10
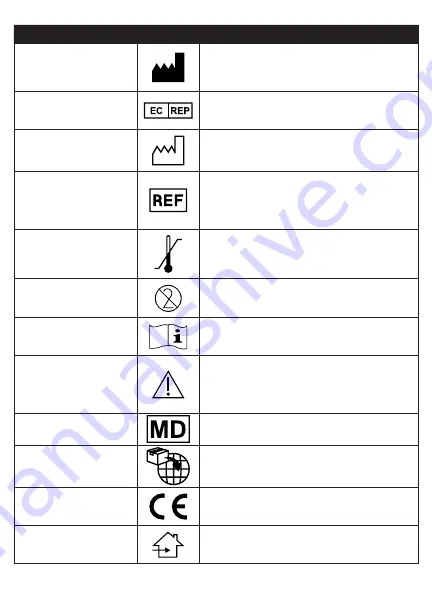
Symbol Glossary
Symbol Title
Symbol
Description and Reference
Manufacturer
Indicates the medical device manufacturer
as defined in Medical Device Regulation (EU)
2017/745 formerly EU Directive 93/42/EEC.
Source: ISO 15223, 5.1.1
Authorized Representative
in European Community
Indicates the authorized representative in the
European Community. Source: ISO 15223, 5.1.2,
2014/35/EU, and/or 2014/30/EU
Date of Manufacture
Indicates the date when the medical device was
manufactured. Source: ISO 15223, 5.1.3
Catalogue number
Indicates the manufacturer's catalogue number so
that the medical device can be identified. Source :
ISO 15223, 5.1.6
Temperature limit
Indicates the temperature limits to which the
medical device can be safely exposed. Source: ISO
15223, 5.3.7
Do not re‑use
Indicates a medical device that is intended for one
use or for use on a single patient during a single
procedure. Source: ISO 15223, 5.4.2
Consult instructions for use
Indicates the need for the user to consult the
instructions for use. Source: ISO 15223, 5.4.3
Caution
Indicates the need for the user to consult the
instructions for use for important cautionary
information such as warnings and precautions that
cannot, for a variety of reasons, be presented on
the medical device itself. Source: ISO 15223, 5.4.4
Medical Device
Indicates the item is a medical device.
Importer
Indicates the entity importing the medical device
into the EU.
CE Mark
Indicates conformity to all applicable European
Union Medical Device Regulations and Directives.
Use Indoors
Indicates medical device be used indoors












































