6.23
Impella
®
System for Use During Cardiogenic Shock
SUMMARY OF SUPPLEMENTAL CLINICAL INFORMATION
Supplemental data was provided to demonstrate safety and effectiveness of the Impella devices
during use. Results from the Impella Registry for the real-world use of the Impella catheters was
provided. The sponsor also provided a benchmark comparison of the Impella Registry data to
a comparable registry dataset for its surgical VAD, the AB5000 Ventricle (PMA approved for a
similar indication). As further evidence, a detailed literature review was provided to support the
overall safety and efficacy of the Impella devices.
RESULTS
The Impella Registry is an ongoing, multi-center, retrospective, observational registry for
collection of de-identified data for patients treated with the Impella 2.5, Impella 5.0 and
Impella LD Support Systems. The registry, which was started by Abiomed in 2009, is open for
participation by qualifying sites in the U.S. and Canada. Since the registry was started to date a
total 59 sites have participated. As of June 30, 2015, there were 40 open sites. The sites include
high and low volume centers, academic (teaching) and non-academic hospitals, public and
private institutions as well as for profit and not for profit centers, almost entirely from the United
States, thus providing a good representation of U.S. clinical practice. In addition, Abiomed used
the Impella Registry as supporting evidence in its original PMA (P140003) application for the
Impella 2.5 System. After reviewing the data, FDA stated (In the PMA’s SSED):
“Use of the device in a comparable patient group, as collected retrospectively via
Abiomed’s USpella (Impella Registry) database, showed results similar to those obtained in
the PROTECT II clinical trial for overall patient outcomes and hemodynamic support during
use.”
The data collection from the Impella Registry includes IRB approval, complete data monitoring,
adverse events (AEs) monitoring and CEC adjudication of AEs. All data is entered electronically
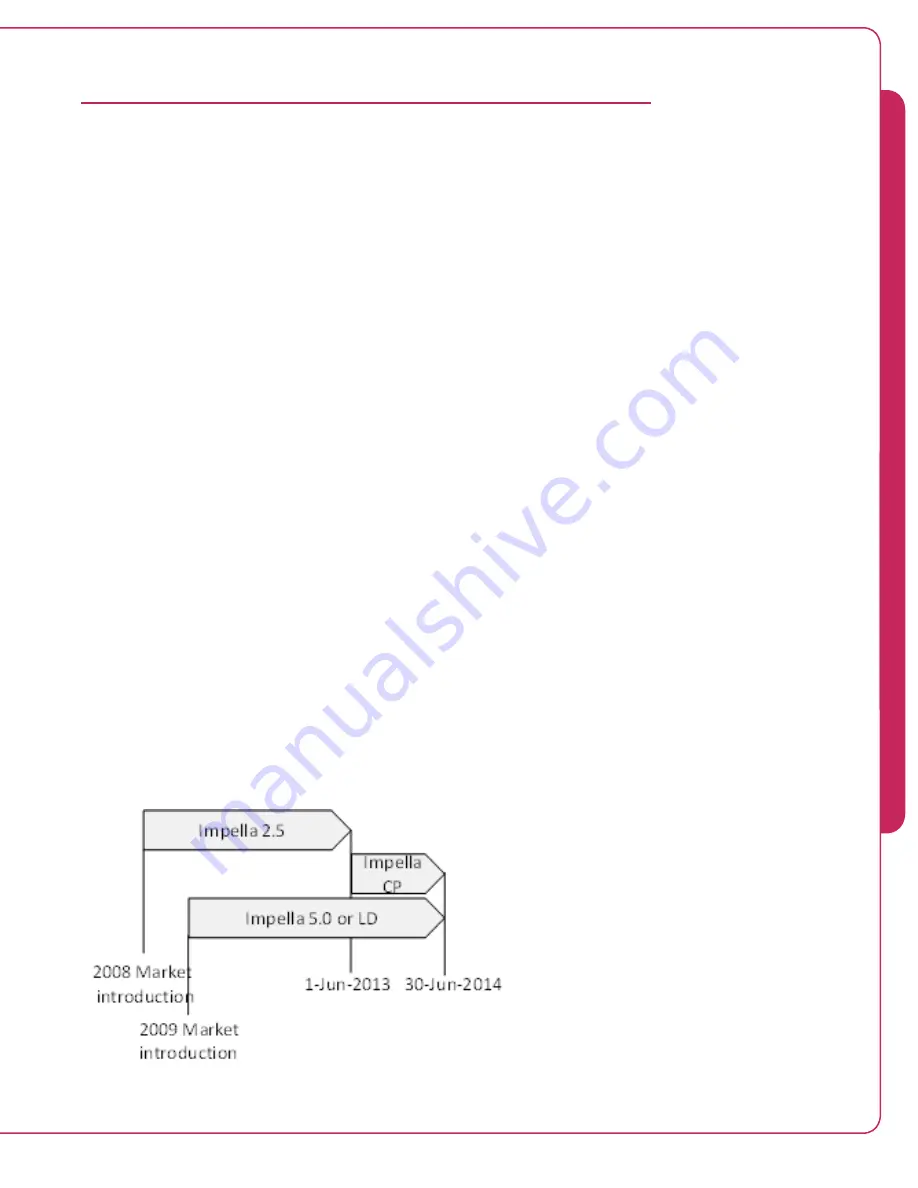
by the sites. For this PMA, the time during which the Impella Registry data was collected is
shown in Figure 6.17. Eligible patients were those who were reported in the Impella Registry,
underwent open-heart surgery and required mechanical circulatory support with Impella devices
within 48 hours post-surgery.
Figure 6.17 Time intervals for Impella implants data collection by type of device
6
C
LIN
IC
A
L E
X
P
E
RI
E
N
C
E
Summary of Contents for Impella 2.5
Page 4: ......
Page 8: ......
Page 10: ......
Page 12: ......
Page 15: ...2 WARNINGS AND CAUTIONS WARNINGS 2 1 CAUTIONS 2 3...
Page 16: ......
Page 22: ......
Page 38: ......
Page 40: ......
Page 108: ......
Page 171: ......
Page 173: ......
Page 181: ......
Page 183: ......
Page 201: ......
Page 203: ......
Page 205: ......
Page 210: ...INDEX TBD...
















































