6.28
Instructions for Use & Clinical Reference Manual (US)
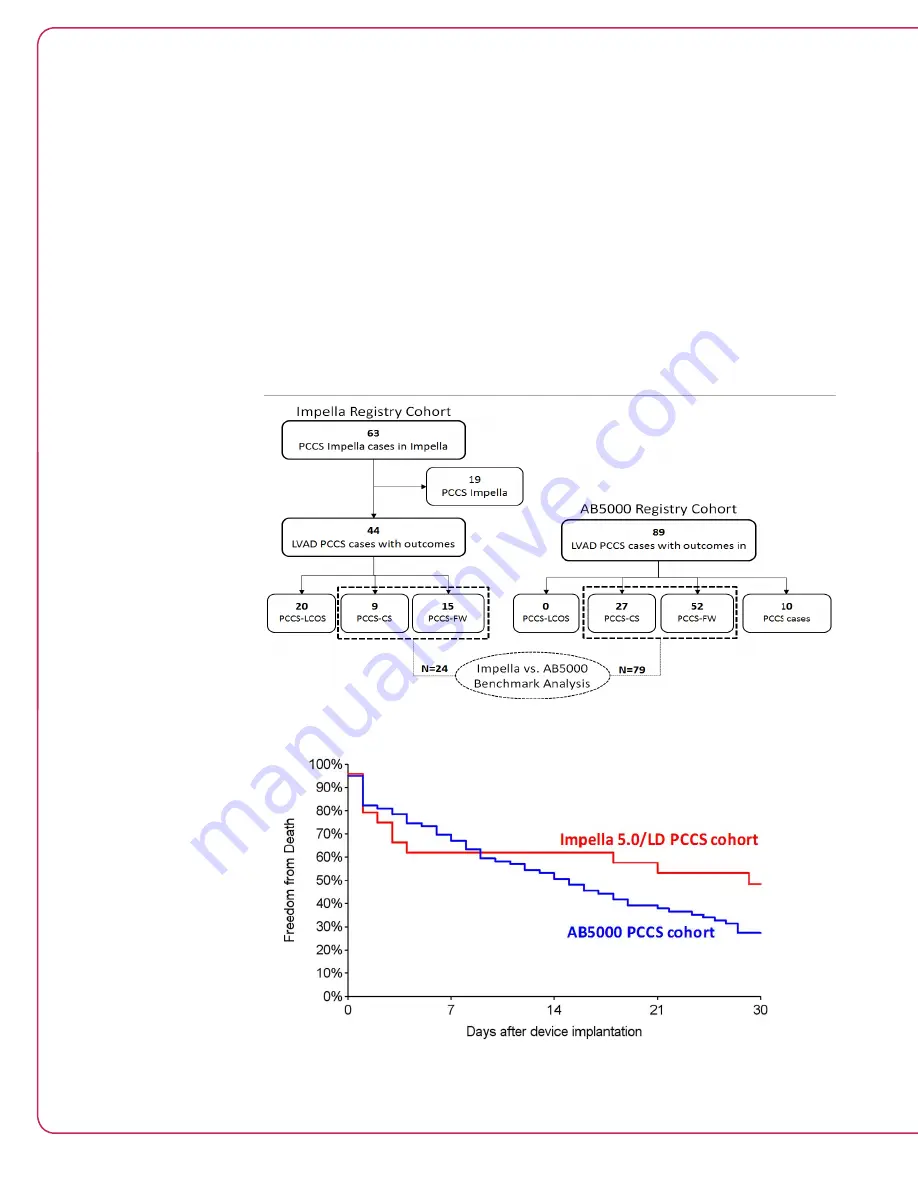
To better match the two cohorts, AB5000 patients who either received bi-ventricular or right
ventricular support were excluded from the benchmark analysis. The AB5000 Registry included
1234 patients (387 of which received only LVAD). Of those patients, 89 were classified as PCCS
patients; however, only 79 cases had enough data to confirm the severity of the presentation
(to serve as the AB5000 benchmark cohort against the Impella Registry cohort). The Impella
Registry benchmark included Impella 5.0/LD patients that presented either with PCCS-CS or
PCCS-FW. The LCOS patients were excluded so the analysis is conservative (considering the
invasiveness of the AB5000, it is very unlikely that the device was used for LCOS patients).
The Impella 2.5 patients were also excluded because it was felt that both the AB5000 and the
Impella 5.0/LD provide full flow as opposed to the Impella 2.5 that provides partial flow (results
of the benchmark analysis of AB5000 vs. Impella 2.5 is provided in the appendix for full access
to the data). The selection of cases for the benchmark comparison is provided schematically in
Figure 6.27.
Figure 6.27 Flow diagram of the distribution of the AB5000 LVAD PCCS patient cohort
Figure 6.28 Kaplan-Meier curve estimates for 30 day survival
Summary of Contents for Impella 2.5
Page 4: ......
Page 8: ......
Page 10: ......
Page 12: ......
Page 15: ...2 WARNINGS AND CAUTIONS WARNINGS 2 1 CAUTIONS 2 3...
Page 16: ......
Page 22: ......
Page 38: ......
Page 40: ......
Page 108: ......
Page 171: ......
Page 173: ......
Page 181: ......
Page 183: ......
Page 201: ......
Page 203: ......
Page 205: ......
Page 210: ...INDEX TBD...
















































