CAUTIONS:
General:
DO NOT store in extremely hot, cold,
humid, wet, or bright conditions.
DO NOT expose to strong electro-
magnetic fields.
DO NOT take recordings in close
vicinity to other equipment emitting
ultrasonic acoustics.
DO keep components out of reach
of children.
DO use this device to record heart
rate and heart rhythm only.
DO not use the sensor on portion
of the body with too much body
fat, body hair or very dry skin, a
successful recording may not be
possible.
AliveCor makes no warranty for any
data or information that is collected
erroneously by the device, or misuse
or malfunction as a result of abuse,
accidents, alteration, misuse, neglect,
or failure to maintain the products as
instructed. Interpretations made by
this device are potential findings, not
a complete diagnosis of cardiac
conditions. All interpretations should
be reviewed by a medical profes-
sional for clinical decision-making.
Kardia Band
DO NOT use with a cardiac pace-
maker, ICDs, or other implanted
electronic devices.
DO NOT continue use until further
instructed by a physician if your skin
is irritated or inflamed around the
sensor or band.
DO NOT drop or bump with excessive
force.
DO NOT use to diagnose heart-
related conditions.
DO NOT wear during magnetic
resonance imaging (MRI), cautery
and external defibrillation procedures.
After ECG analysis, the app may
incorrectly identify ventricular flutter,
ventricular bigeminy, and ventricular
trigeminy heart conditions as
unreadable. Please consult with
your physician.
CAUTION
: AliveCor does not
guarantee that you are not experi-
encing an arrhythmia or other health
conditions when labeling an ECG
as normal. You should notify your
physician for possible changes in
your health.
KARDIA BAND
SPECIFICATIONS
Battery____________ Coin Cell
Storage Conditions_______
Original package under normal
room temperature and humidity
ELECTROMAGNETIC & OTHER
INTERFERENCES
The Kardia Band has been tested
and deemed in conformance with
the relevant requirements in
EN60601-1-2:2007 Class BF for
Electromagnetic Compatibility (EMC).
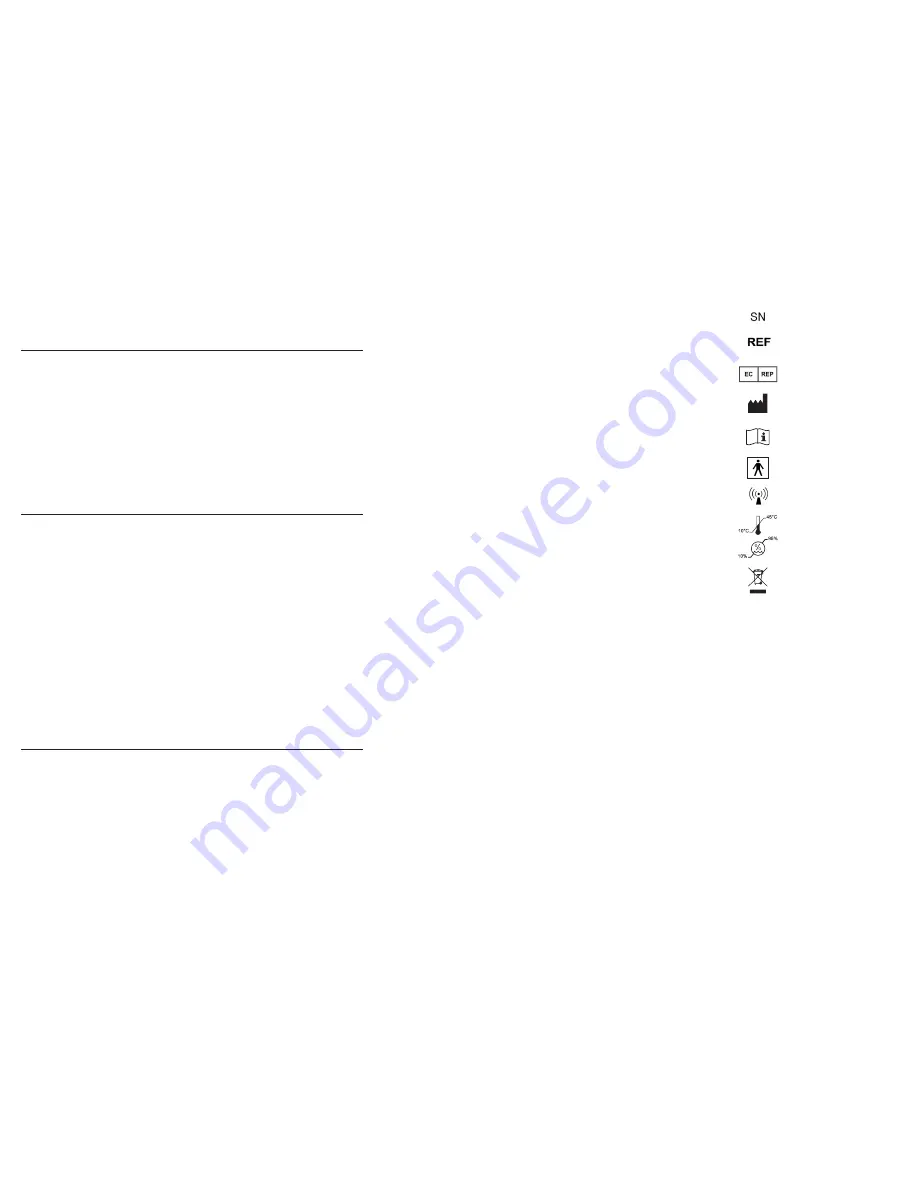
EQUIPMENT SYMBOLS
Serial number
Model number
European Authorized
Representative
Manufacturer
Read instructions
before use
Type BF applied part
Emits radio waves
Temperature range
Humidity range
Do not dispose with
household waste
ADDITIONAL INFORMATION
For more detailed troubleshooting
and technical information, please
visit: https://www.alivecor.com/
support/#user-manual
Problem:
My Kardia Band is not working.
Problem:
I have a lot of artifact, noise, or
interference in my recording, or “No
EKG recorded” message displays.
Problem:
The ECG rhythms appear
upside down.
TROUBLE SHOOTING
If you experience difficulties in operating your AliveCor products, refer
to the troubleshooting guide below or contact technical support at
support@alivecor.com.
Solution
Option 1:
Ensure that the Kardia
watch app has access to the watch’s
microphone. On the iPhone, go to
Settings and tap the Kardia app.
Tap the microphone toggle.
Option 2:
Ensure that the watch
microphone is unobstructed.
Consult the watch user manual if
it is obstructed.
Solution
Option 1:
Ensure that your watch,
arms, and hands remain still during
recordings.
Option 2:
Clean the electrodes on
the Kardia Band with an alcohol-
based sanitizer.
Option 3:
If your hands are very
dry, use a water-based lotion before
recording.
Option 4:
When recording, relax
your arms and hands to reduce
muscle noise. Rest the forearms
and hands on a flat surface.
Solution
Option 1:
The watch orientation
may be set to the wrong wrist. On
your iPhone, go to the Watch app.
Tap My Watch > General > Watch
Orientation.
Option 2:
The Kardia Band pieces
may be attached to the watch in the
wrong orientation. Review “Assembly”
instructions.




















