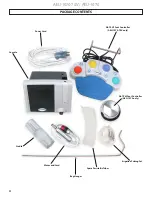
2
AEU-1070-70V / AEU-1070
!
SAFETY PRECAUTIONS
Aseptico accepts no liability for direct or consequential injury or dam-
age resulting from improper use, arising in particular through the
non-observance of the operating instructions, or improper preparation
and maintenance of this product.
To prevent injury to people and damage to property, please
heed relevant warnings and remarks. They are marked as follows:
• WARNING
: Serious injury or death may result if ignored.
• CAUTION
: Damage to property or the environment may re-
sult if ignored.
• NOTE
: Important additional information and hints.
s
WARNING:
The system is supplied non-sterile! Before first use,
and before each patient use thereafter, sterilize specified com-
ponents as recommended in the Cleaning, Maintenance and
Sterilization section.
s
WARNING:
Use for intended purposes only. Failure to observe
the operating instructions may result in the patient or user suf-
fering serious injury or the product being damaged, possibly
beyond repair. Before using this product, make sure that you
have studied and understood the operating instructions.
s
CAUTION:
Federal law restricts this device to sale by or on the
order of a dentist.
s
CAUTION:
Use of other dental accessories or sub-assemblies
from third-party manufacturers is the sole responsibility of the
user.
s
CAUTION:
All repairs are to be performed by authorized service
personnel only.
s
CAUTION:
Always follow these guidelines when operating the
unit:
• Never touch drills, burs, files, or other handpiece tips when
they are still rotating.
• The handpiece should only be attached when the motor has
stopped running.
s
WARNING:
Do not install where there is a risk of an explosion.
The system is not intended for operation in the presence of
flammable anesthetics or gases.
s
WARNING:
All handpieces have inherent inefficiencies that can
lead to torque variations. Routine calibration is recommended,
even if using the same handpiece or whenever a handpiece is
changed. If further verification of torque accuracy is desired,
then it is suggested that a torque wrench be used.
s
WARNING:
Always comply with the handpiece and implant/
file manufacturers’ instructions regarding maximum speeds,
torques, forward and reverse directions, and use of all instru-
mentation, drills, burs, etc., used in endodontics, implantology,
and other oral surgery applications.
s
CAUTION:
The irrigation supply system is designed for use with
a saline solution or sterile water. For implants, use only suitable
irrigants as recommended by the manufacturer’s instructions.
s
CAUTION:
Connect mains power cable to a properly grounded
outlet only.
s
CAUTION:
The motor is sensitive to shock and may be damaged
if dropped or impacted against a hard surface.
s
WARNING:
Do not disassemble or alter the system motor, con-
sole, or foot switch. No modification of the unit is allowed.
s
CAUTION:
Use only appliance cord Type C13, 10A per IEC / EN
60320-1. Note: North America, Denmark, Australia, and New
Zealand may require hospital grade plugs. Consult local codes.
s
WARNING:
Do not use this device in conjunction with an elec-
tric scalpel or on patients with pacemakers.
s
CAUTION:
Never connect or disconnect the bag spike to the
irrigation bag over the console. Water spilled onto the console
can damage the unit.
s
CAUTION:
It is recommended to always have the patient wear a
rubber dam during implant procedures.
s
WARNING:
Using a different handpiece model from those rec-
ommended can cause significant under- or over-torque.
s
WARNING:
Using the incorrect ratio setting can cause signifi-
cant under- or over-torque and under- or over-speeds.
s
WARNING:
Do not position the unit so that the power cord is
inaccessible for disconnection during use.
!
DANGER
RISK OF EXPLOSION IF
USED IN THE PRESENCE OF
FLAMMABLE ANESTHETICS
Risque d’explosions’il est utilisé avec
les anethésiques inflammables
!
This device has been tested and found to comply with the
emissions requirements of IEC 60601-1-2:2001-09. These require-
ments provide reasonable protection against harmful electro-
magnetic interference in a typical medical installation. However,
high levels of radio-frequency (RF) emissions from electrical
devices, such as cellular phones, may disrupt the performance of
this device. To mitigate disruptive electromagnetic interference,
position this device away from RF transmitters and other sources
of electromagnetic energy.
Information concerning the accuracy and precision of this product
may be obtained upon request by contacting Aseptico at the ad-
dress shown on this page.
P.O. Box 1548, Woodinville, WA 98072
8333 216th Street S.E., Woodinville, WA 98072
Phone (425) 487-3157 • Toll Free (800) 426-5913
Web: www.aseptico.com
Email: info@aseptico.com