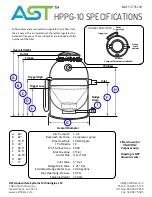
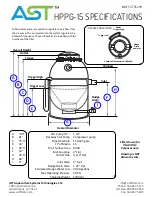
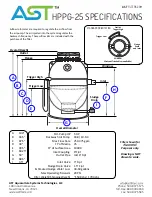
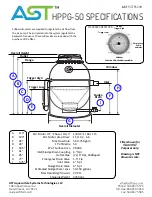
21
1.
You may have air bubbles in your recirculating flow. These bubbles are moving up
through the bed dislodging fine solids. Check the suction side of the pump for air leaks.
2.
Try decreasing your backflush frequency. This will increase your internal settling time
between backwash events and improve the fine solids capture of the bead bed.
Low pH
The pH in an RAS will naturally decline as nitrifiers convert TAN to nitrite. Bicarbonates are gradually
consumed by the nitrifiers and both the fish and bacteria excrete carbon dioxide that tends to lower pH.
If your pH is below 7.0, increasing alkalinity is warranted immediately to prevent TAN or nitrite
accumulations.
If the alkalinity is below 100 mg-CaCO
3
, add sodium bicarbonate or soda ash until alkalinity
exceeds 150 mg-CaCO
3
.
Increase aeration to the system. This should lower the carbon dioxide and raise the pH.
Elevated Ammonia Levels
Elevated levels of Nitrite may occur if the dissolved oxygen concentration in the effluent leaving
the filter drops below 2 mg/l. Low DO concentrations leaving the filter can often be solved by
increasing the dissolved oxygen levels in the tank/pond or through increased aeration or by
increasing the flow rate through the filter.
Elevated Nitrite levels may also occur if your total alkalinity (as CaCO
3
) drops below 80mg/l. We
recommended you maintain your alkalinity at 100-200 mg/l as CaCO
3
at all times. If you
experience low alkalinity, simply add baking soda to the filtration system periodically to
maintain proper levels. Sodium bicarbonate is not suitable for aquaponics applications because
sodium can harm plant development and growth. Applications of different liming agents, such
as potassium hydroxide (KOH) and Ca(OH)2 are recommended for balanced plant growth.
Elevated Nitrite levels can also occur from over washing the bead bed. If the flow rate, effluent
oxygen and alkalinity are satisfactory, the backwash frequency can simply be reduced. This
situation typically occurs when you go from periods of high loading and frequent backwashing to
periods of reduced loading with frequent backwashing.
Although generally the nitrification can be achieved across a wide ban of backwash intervals,
backwashing for cold water systems must be more carefully managed. Generally, backwashing
should be limited to a low frequency (<1 day) for cold water applications. The reduced
frequency allows more time for the slow growing NOBs to recover from backwashing biofilm
damage.
Sulfide Production
Sulfur is normally found in a surface water in the form of sulfates (SO4
=
) an inert compound that has
little impact on aquatic species. If the oxygen is removed from the water, then the sulfates are