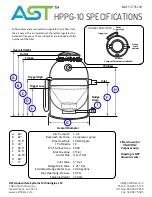
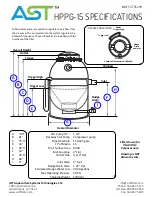
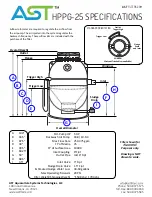
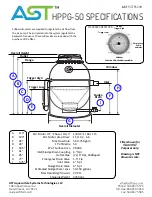
31
hydrogen ions (H
+
) in the water to form the ammonium ion (NH
4
+
) and the combination (NH
3
+NH
4
+
) it
referred to as Total Ammonia Nitrogen or TAN. Although only the free ammonia is toxic to fish, it is the
TAN concentration that controls the bacterial conversion rates. The heterotrophic bacteria initiate the
degradation process by attaching the hydrogen and carbon bonds that characterize and organic waste.
This an oxidation step that releases ammonia whenever a nitrogenous compound is encounters.
Heterotrophic bacteria use oxygen and release carbon dioxide, but, have little effect pH or the
bicarbonates in the system. The AOBs in system then initiate the first step in the nitrification process by
the oxidation of TAN to nitrite. This process consumes both oxygen and bicarbonates while producing
Nitrite and carbon dioxide. The second groups of nitrifying bacteria, the NOBs, then oxidize the Nitrite
to the non-toxic Nitrate (NO
3
-
).