12
7-4
Packaging, Sterilizing, and Drying
1) Insert the motor into an FDA-approved sterilization pouch
that conforms to ISO 11607-1 (EN ISO 11607-1), and seal
the pouch.
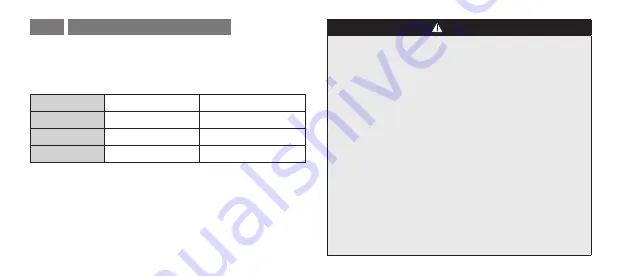
2) Perform steam sterilization with the following conditions.
Type
Gravity Displacement Dynamic Air Removal
Temperature
270°F (132°C)
270°F (132°C)
Full Cycle Time 15 min
4 min
Drying Time
30 min
30 min
CAUTION
• Use an FDA-approved steam sterilizer to perform
sterilization.
• Follow local rules, regulations, and guidelines regarding the
reprocessing of devices.
• Do not touch the product immediately after steam
sterilization as it will be very hot and must remain in a
sterile condition.
• Do not perform steam sterilization on the product with
other instruments even when it is in a pouch. This is to
prevent possible discoloration and damage to the product
from chemical residue on other instruments.
• Clean and lubricate the motor prior to sterilization. If blood
remains on the internal surface it can become clotted and
cause product failure.
• Do not heat or cool the product too quickly. Rapid change
in temperature could cause damage to the product.
Summary of Contents for A-dec 301
Page 19: ......






































