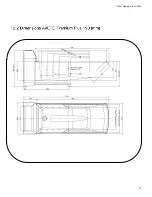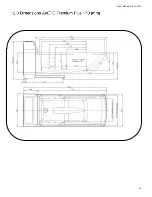
Prism Medical Ltd. V1-2015
39
Electrical medical equipment needs to be installed with special precautions taken regarding
Electromagnetic Compatibility (EMC) and must be installed and put into operation according to
EMC information contained in the accompanying documents.
No special measures must be taken for devices and systems of BEKA Hospitec Ltd.
Portable and mobile Radio Frequency (RF) communications equipment can affect electrical
medical equipment.
Guidelines and manufacturer declaration - electromagnetic immunity
The AVERO Premium Plus is intended for use in the ELECTROMAGNETIC ENVIRONMENT
specified below. The customer or user of the AVERO Premium Plus should ensure that it is used
in such an environment.
Release measurements
Agreement
Electromagnetic environment - guidelines
High-frequency (RF) emissions
according to
CISPR 11
Group 1
The AVERO Premium Plus uses RF energy for its
internal functions only. Therefore, the RF radiation of
the device is very low and it is unlikely that nearby
electrical devices will be disturbed.
High-frequency (RF) emissions
according to
CISPR 11
Class B
The AVERO Premium Plus is intended for use in all
facilities including residential areas and the like that are
directly connected to a public power supply that also
supplies buildings used for domestic purposes.
Harmonics according to
IEC 61000-3-2
Class A
Voltage fluctuations /
flickers according to
IEC 61000-3-3
complies
Summary of Contents for 810142501
Page 1: ...1 Plumbing CSA B45 5 11 IAPMO 7124 2011 Electrical CSA C22 2 No 60601 1 ...
Page 6: ...Prism Medical Ltd V1 2015 6 2 Introduction WARNING ...
Page 31: ...Prism Medical Ltd V1 2015 31 11 Troubleshooting and customer service Problem Solution ...
Page 35: ...Prism Medical Ltd V1 2015 35 ...
Page 36: ...Prism Medical Ltd V1 2015 36 ...
Page 37: ...Prism Medical Ltd V1 2015 37 ...