KBF-LQC (E5.2) 12/2010
page 72/106
15. ICH compliant illumination according to CPMP/ICH/279/95 (Q1B)
15.1 BINDER ICH light
Drugs are tested according to extensive test procedures and only thereafter are admitted for distribution.
Part of the approval procedure is the proof that the products do not or only minimally change within the
serviceable life. One of the tests to be executed is the photostability test according to ICH guideline Q1B.
For this test product samples must be exposed to a quantity of light of at least 1.2 million LUX x hours in
climatic chambers with ICH compliant illumination. To prove the quantity of light a temporal integration of
illumination (LUX) and UV intensity (W/m²) e.g. by optical sensors is needed.
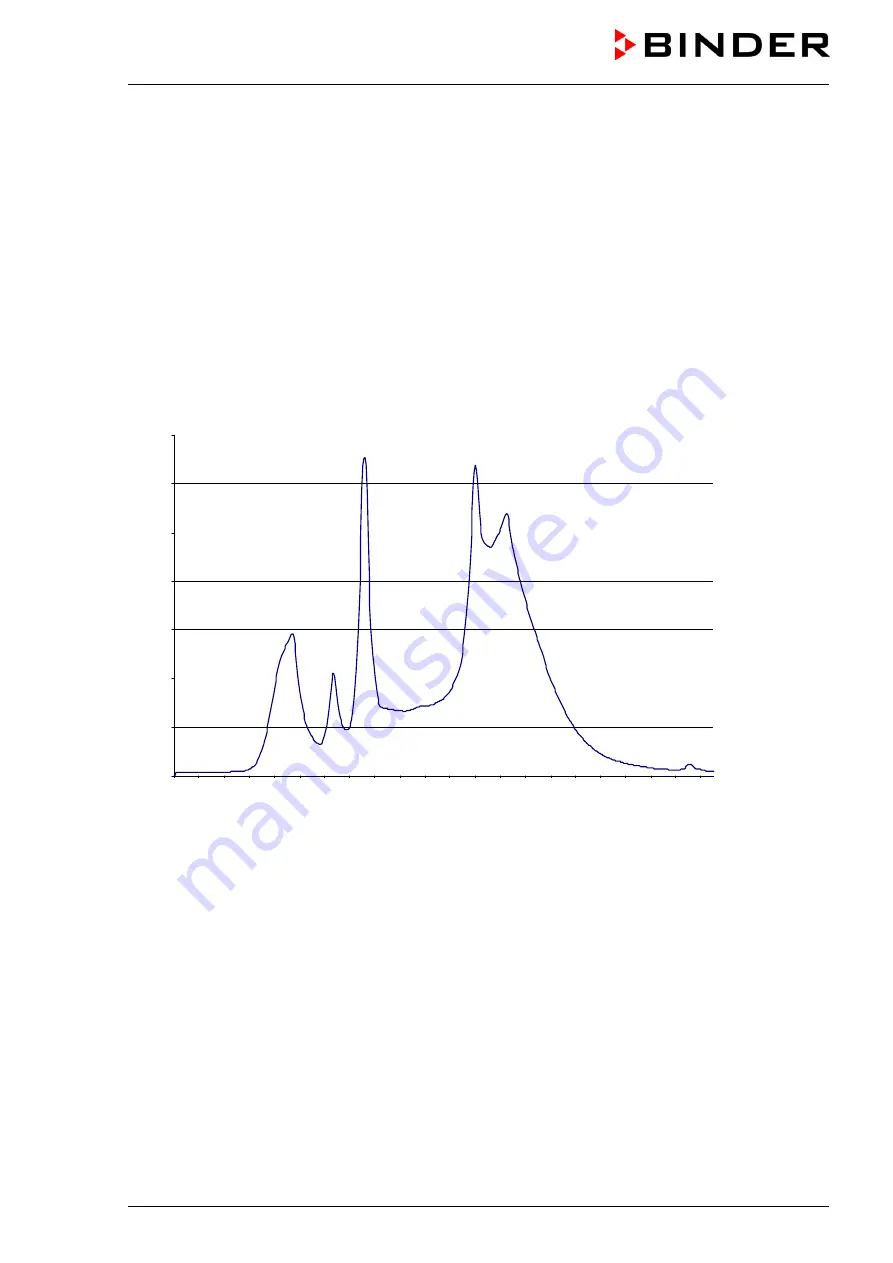
Beside pure cool white fluorescent tubes (light color 640) the special fluorescent tubes “BINDER Q1B
Synergy Light” available only at BINDER are used, which combine emission of both radiations UVA (light
color 09) and cool white (light color 640). Combination of these tubes leads to a spectral distribution ac-
cording to option 2 of guidelineCPMP/ICH/279/95 (Q1B).
0
5000
10000
15000
20000
25000
30000
35000
250
275
300
325
350
375
400
425
450
475
500
525
550
575
600
625
650
675
700
725
750
775
Figure 24: Overall spectrum cool white light color 640 and UVA light color 09
Advantages of the BINDER light system:
•
Reaching the radiation doses of UVA and LUX requested by Q1B almost simultaneously.
•
After having reached the target intensity of guideline CPMP/ICH/279/95 (Q1B), you can turn off the
fluorescent tubes with a UVA portion (BINDER Q1B Synergy Light) independently from the tubes with
visible spectral range.
•
Optimum homogeneity of the spectral distribution and the intensities in LUX and UVA on the shelf
surface, even with high intensity values, obtained by the BINDER ICH light and the special lens of the
headlight. This guarantees that all samples receive the same radiation doses, thus permitting very
precise test conditions for photo stability tests.






























