________________________________________________________________
___
54
English
Issue 02 - 07/2001
Kinetics
The usual way of measuring the rate of an enzyme reaction is to monitor the change
in concentration of one of the substrates involved in, or of one of the products
produced by, the reaction. Take for example the alanine transaminase (ALT) enzyme
reaction: -
ALT
α
–oxoglu alanine
=
py glutamate.
If we want to measure the rate of production of pyruvate, then as this cannot be done
directly we can link it to another enzyme reaction involving NADH and the enzyme
lactate dehydrogenase (LDH). Thus: -
LDH
py NADH + H
+
=
l NAD
+
We can follow the rate at which NADH is used up by measuring the absorbance of
the reaction mixture at 340 nm. Since LDH is in excess, this rate is directly
proportional to the rate of pyruvate produced in the first reaction (about 80% of all
enzyme measurements are monitored in this way).
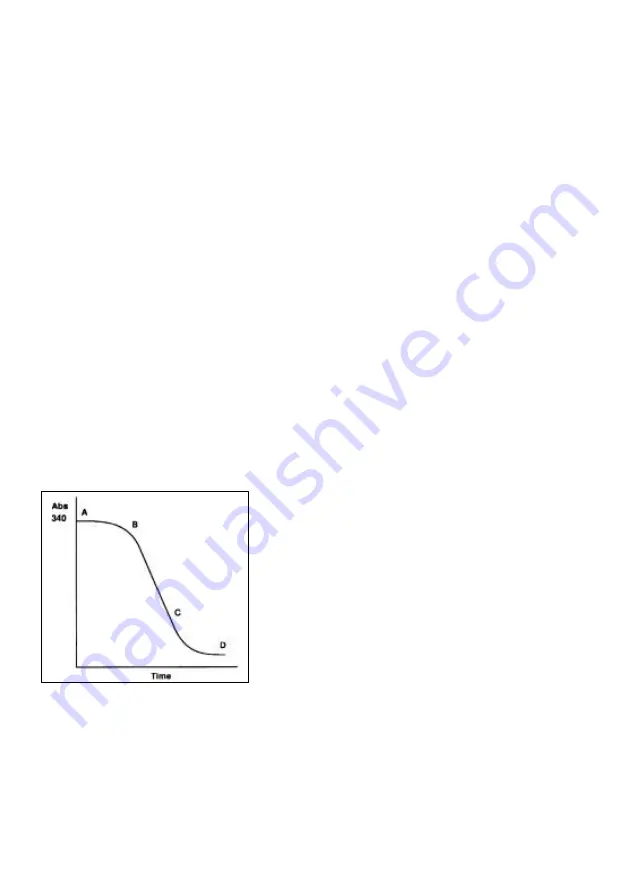
If a curve of the absorbance of the reaction mixture at 340 nm versus time is plotted, a
graph similar to that illustrated will be obtained.
The curve can be split into 3 phases :-
Phase 1, A–B; reactant mixing, thermal
equilibrium and attainment of linear phase,
Phase 2, B–C; the linear phase,
Phase 3, C–D; tail off as one of the reactants
becomes rate limiting, thus reducing the net
reaction rate to zero.
The rate of the reaction is defined by the slope of the linear portion of the plot and
therefore, from Beers Law, the change in absorbance per unit time, dA/dt, is given
by:
dC/dt
= (dA /dt) x (1/EL)







































