4
System Overview
Device family
This device family consists of single-chamber, dual-chamber and triple-chamber
devices with or without Home Monitoring. Not all device types are available in every
country.
The following device variants are available:
Device
The device's housing is made of biocompatible titanium, welded from the outside and
therefore hermetically sealed. The ellipsoid shape facilitates ingrowth into the pectoral
muscle area. The housing serves as an antipole in the case of unipolar lead configura-
tion.
IS-1 lead connection
The device labeling provides information pertaining to the connection assignment:
Leads
BIOTRONIK leads are sheathed in biocompatible silicone. They can be flexibly maneu-
vered, are stable long-term, and are equipped for active or passive fixation. They are
implanted using a lead introducer set. Some leads are coated with polyurethane to
increase the gliding properties of the lead. Leads with steroids reduce inflammatory
processes. The fractal design of the leads allows for low pacing thresholds, high pacing
impedance, and a low risk of oversensing.
BIOTRONIK provides adapters to connect already implanted leads to new devices.
Telemetry
Telemetric communication between the device and the programmer can be carried out
following initialization either by applying the programming head (PGH) to the device or
by using radio frequency (RF) telemetry in the programmer. BIOTRONIK calls this
function SafeSync
®
.
Programmer
Implantation and follow-up are performed with BIOTRONIK's portable programmer:
There are programmers with integrated or external SafeSync Module for RF telemetry.
The programmer is used during implantation to transfer the current device program to
the device. The pacing thresholds can be determined and all tests can be performed
during in-office follow-up. In addition to this, the programmer is used to set mode and
parameter combinations, as well as for interrogation and saving of data from the
device. Leadless ECG, IEGM, markers and functions are displayed simultaneously on
the color display.
Modes
The mode setting depends on the individual diagnosis:
Device type
Variant with
Home Monitoring
Variant without
Home Monitoring
Single-chamber
Eluna 8 SR-T
Eluna 8 SR
Dual-chamber
Eluna 8 DR-T
Eluna 8 DR
Triple-chamber
Eluna 8 HF-T
—
SR
DR
HF
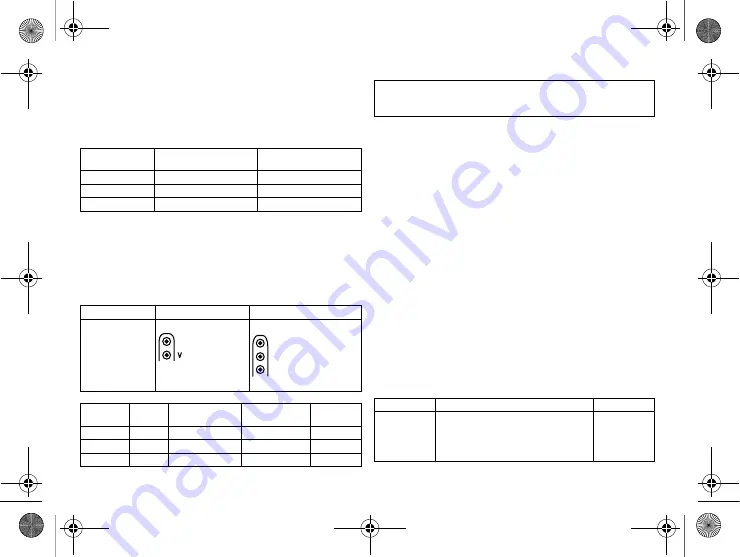
Connector
port
Lead
connector
Configuration
Implantation site
Device type
RA
IS-1
Unipolar, bipolar
Atrium
DR, HF
RV
IS-1
Unipolar, bipolar
Right ventricle
SR, DR, HF
LV
IS-1
Unipolar, bipolar
Left ventricle
HF
VVIR
/AAIR
IS-1
DDDR
A
IS-1
DDDR
A
IS-1
V
LV
RV
Note:
Use only adapters approved by BIOTRONIK for leads with different connections.
• If you have any questions concerning the compatibility of other manufacturers'
leads, please contact BIOTRONIK.
Device type
Modes
Standard
SR(-T)
• VVI-CLS (8 series only)
• VVIR; V00R; AAIR; A00R
• VVI; VVT; V00; AAI; AAT; A00
• OFF
VVIR
401303--G_GA_Eluna-I-ProMRI_mul.book Page 4 Friday, October 2, 2015 4:57 PM






































