en • English
5
• An additional diagnostic function with biventricular pacing: variability of the heart
rate, the patient activity and the thoracic impedance are monitored on a continual
basis.
Programs
There are two types of therapy programs:
• Default parameters are offered for the most common indications (Program Consult
function).
• Individual settings can be saved in three individual therapy programs.
Home Monitoring functions
The device automatically sends information to the transmitter once a day. Additionally,
the test messages can be initiated using the programmer. Important medical informa-
tion include, among others, the following:
• Ongoing atrial and ventricular arrhythmia
• Parameters relevant to leads in the atrium and ventricle: pacing thresholds,
sensing amplitudes, impedances
• Current statistics on bradycardia therapy
• Individually adjustable timing interval for device messages which provide additional
information pertaining to the device messages
• IEGM online HD
®
with up to three high definition channels
• Sending of these IEGM recordings with device messages
Package Contents
Standard
The storage package includes the following:
• Sterile packaging with device
• Serial number label
• Patient ID card
• Warranty booklet
• Technical manual for the device
The sterile container includes the following:
• Device
• Screwdriver
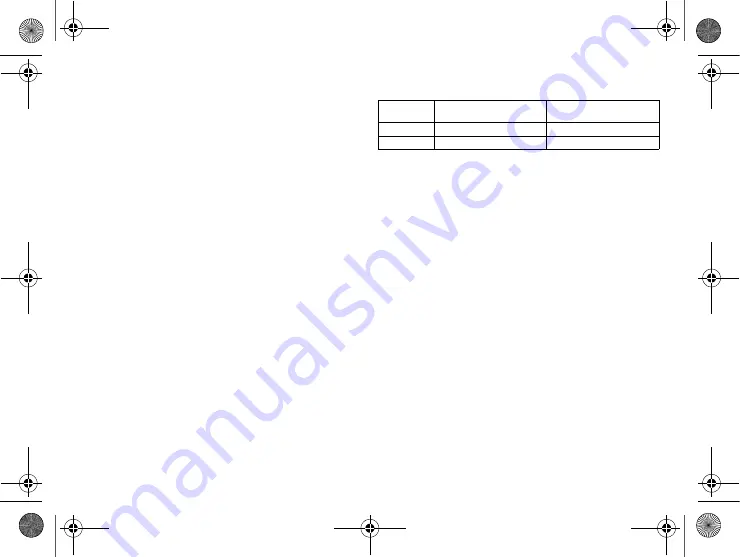
Order numbers Evia
The devices can be obtained as follows:
2 General
Safety
Instructions
Possible Medical Complications
General information on medical complications
Complications for patients and device systems generally recognized among practitio-
ners also apply to BIOTRONIK devices.
• Normal complications may include fluid accumulation within the device pocket,
infections, or tissue reactions. Primary sources of complication information include
current scientific and technological knowledge.
• It is impossible to guarantee the efficacy of antitachycardia therapy, even if the
programs have proven successful during tests or subsequent electrophysiological
examinations. In rare cases the set parameters may become ineffective. In partic-
ular it cannot be excluded that tachyarrhythmias may be induced.
Skeletal myopotentials
Bipolar sensing and control of sensitivity are adapted by the device to the rate range of
intrinsic events so that skeletal myopotentials are usually not sensed. Skeletal myopo-
tentials can nonetheless be classified as intrinsic events especially with a unipolar
configuration and/or very high sensitivity and, depending on the interference, may
cause inhibition or antiarrhythmia therapy.
Nerve and muscle stimulation
A device system consisting of a unipolar lead and an uncoated device may result in
undesirable pacing of the diaphragm in the case of an initial or permanent high setting
of the pulse amplitude.
• BIOTRONIK also provides coated devices.
Device
Order number:
uncoated
Order number:
coated
HF-T
381534
381535
HF
381532
381533
387513--D_GA_Evia-HF-ProMRI_mul.book Page 5 Thursday, September 12, 2013 3:34 PM







































