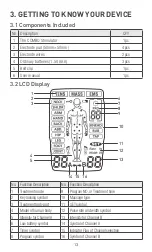
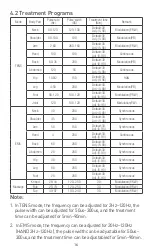
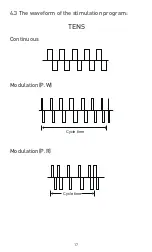
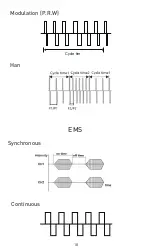
8
2.2.2 Warning
1) The long-term effect electrical stimulation is unknown.
Electrical stimulation device, do not have any curative value.
2) This device should only to be used under the continued
supervision of a licensed medical practitioner.
3) Electrical stimulation is not effective for central origin pain
such as headache.
4) Stimulation should not be applied over the carotid sinus
nerves, particularly in patients with a known sensitivity to the
carotid sinus reflex.
5) Stimulation should not be applied over the neck or mouth.
Severe spasm of the laryngeal and pharyngeal muscles may
occur and the contractions may be strong enough to close
the airway or cause difficulty in breathing, or adverse effects
on heart rhythm or blood pressure.
6) Do not apply stimulation in the presence of electronic
monitoring equipment (e.g., cardiac monitors, ECG alarms),
which may not operate properly when electrical stimulation
device is in use.
7) Do not apply stimulation across the patient’s chest because
the introduction of electrical current into the chest may cause
rhythm disturbances to the patient’s heart, which could be
lethal.
8) Do not apply stimulation over open wounds or rashes, or over
swollen, red, infected, or inflamed areas or skin eruptions (
e.g. phlebitis, thrombophlebitis, varicose veins).
9) Do not apply stimulation while the patient is sleeping.
10) Stimulation should not be applied transthoracically in that
the introduction of electrical current into the heart may cause
cardiac arrhythmias.