18
Questions about Cala Trio? Visit CalaTrio.com/HCPFAQs
LBL-5122 Rev C NOV 2019
»
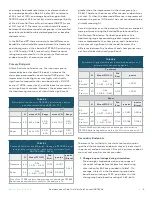
Responder Rate
Additionally, the responder rate by therapy
allocation, was calculated for each of the TETRAS
tasks, where the responder rate was defined as the
percentage of subjects with a ≥ 0.5 point improvement
from baseline.
9.5 Protocol
For each subject’s single in-clinic visit, baseline
measurements of the study effectiveness endpoints
were taken prior to stimulation with treatment or sham.
After 20 seconds at a specific stimulation level, the device
automatically transitioned into a 40-minute stimulation
session of treatment with TAPS
(Cala ONE device will
continue stimulating at the same level)
or sham
(Cala
ONE device will transition to 0 amplitude stimulation)
.
The device continued operating for 40 minutes. Endpoint
measurements were taken during and after stimulation.
During stimulation the subject repeated the same set of
TETRAS tasks that were performed during baseline, at 30
+/- 5 minutes into the session. A study-trained neurologist
rated all performed TETRAS tasks in-person, except for
the Archimedes spiral task, which was rated later by
blinded raters.
After the 40-minute stimulation session, the Cala ONE
device automatically turned off. With the Cala ONE
device remaining on the subject’s wrist, the neurologist
instructed each subject to repeat the same set of TETRAS
tasks. The neurologist rated all performed TETRAS tasks
in-person, except for the Archimedes spiral task, which
was rated later by blinded raters.
Next the subject repeated the same Bain & Findley ADLs
completed during baseline, and rated themselves on
each task. The subject also assessed any changes in their
tremor level
(compared to baseline)
using the Clinical
Global Impression– Improvement (CGI-I) scale.
For the effectiveness endpoints and to assess whether the
subject met the criteria to be included in the Effectiveness
Analysis Population
(see below)
, 3 independent blinded
raters evaluated the Archimedes spirals collected at
baseline, during, and after stimulation as described above.
The raters were board certified neurologists trained in
movement disorders, and were blinded to the therapy
allocation
(sham or treatment)
and to the spiral order
(e.g., baseline, during, or after stimulation)
. The
independent blinded raters rated the spirals using the
5-point
(0-4)
TETRAS scale using a 0.5-point resolution.
The scores from all three raters were averaged to get the
final rating for each spiral.
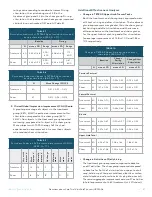
9.6 Statistical Analysis Plan (SAP)
•
Analysis populations
»
The primary and secondary effectiveness endpoints
were assessed on the
Effectiveness Analysis
Population (EAP)
, which was defined as the enrolled
subjects with a baseline TETRAS spiral rating ≥ 2 as
assessed by the average score from 3 independent
blinded raters.
»
Per protocol (PP)
analysis set included subjects
who had no major protocol deviation and was
done as sensitivity analysis for primary and
effectiveness endpoints.
»
Safety analysis
included all enrolled subjects.
•
Safety Analysis
Adverse event (AE) rates were planned to be presented
on all enrolled subjects, overall as well as by treatment
group. The rates of events and type were presented and
compared between groups using the Fisher’s Exact test.
•
Blinding Assessment
The successfulness of the blinding of subjects was
assessed at the end of the study visit using a blinding
assessment questionnaire. Subjects were asked whether
they thought they were in the active or sham group or
if they do not know on a three-point scale. The sponsor
calculated the distribution of the responses to this
assessment.
9.7 Study Results
Subject Disposition
The first subject was enrolled on 11-Apr-2016 and the
last subject completed on 4-Nov-2016. The subject
disposition is provided in Figure 9. A total of 111 subjects
were screened for the study, and 93 subjects were enrolled
and randomized. 48 subjects were randomized to receive
TAPS stimulation
(“treatment” group)
, and 45 subjects
were randomized to receive 0-amplitude sham stimulation
(“sham” group)
. 92 of the 93 enrolled subjects completed
the study; one subject discontinued because the subject’s
wrist circumference was outside the range of wrist
circumferences for which the Cala ONE is designed. Of the
92 subjects who completed the study, 77
(37 in the sham
group and 40 in the treatment group)
met the pre-specified
EAP criteria of having a baseline TETRAS Archimedes
spiral rating ≥ 2, as assessed by the three blinded raters.
As prespecified in the investigational plan, effectiveness
was assessed on the Effectiveness Analysis Population,