23
Questions about Cala Trio? Visit CalaTrio.com/HCPFAQs
LBL-5122 Rev C NOV 2019
9.8 Adverse Events
No Serious Adverse Events (SAEs) or Unanticipated
Adverse Device Effects (UADEs) were reported. Of the
93 enrolled subjects, there were 4 non-serious adverse
events amongst 3 subjects. The 4 adverse events were
mild, anticipated, and resolved within 24 hours without
any intervention or sequelae:
»
One subject (Treatment)
reported 2 adverse events:
- a feeling of weakness in the wrist with stimulation
- skin irritation
During the stimulation session, the subject reported
a sensation of weakness around the treated wrist.
The sensation resolved after the session with no
intervention and no sequelae. After the device was
removed, the subject noted skin redness in the area
where the gels were adhered. The redness resolved
the same day with no intervention and no sequelae.
»
A second subject (Treatment)
reported skin irritation,
which was described as swelling in the stimulated
hand. The adverse event was reported the day
following the stimulation session. The swelling
resolved the next day with no intervention and no
sequelae.
»
A third subject (Sham)
reported stinging pain in the
wrist during the sham stimulation session. The subject
requested that the stimulation level be decreased,
thus the sham stimulation level was simulated to be
“decreased” from 3.75 mA to 3.5 mA as displayed on
the device, but the device remained at 0-amplitude
stimulation during the entire 40-minute session. The
subject reported that the stinging sensation was
tolerable once the stimulation level was decreased,
and the sensation had completely resolved when
the device was removed. The incident was resolved
without sequelae.
During active enrollment, the study was monitored by an
independent Safety Reviewer who is a board-certified
neurologist and movement disorder specialist. The Safety
Reviewer could make recommendations for protocol
modifications or trial discontinuation for safety-related
reason. However, during the study, there were no such
recommendations made. On 1 December, 2016 (after
all subjects had been exited from the study), the Safety
Reviewer reviewed the adverse events (AE) and confirmed
the Investigator’s assignment of AE classification, device
relatedness and severity for all 4 events described.
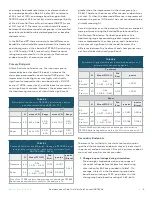
TABLE 14
Adverse Event Rates
All (N=93)
Treatment
(N=48)
Sham
(N=45)
Any adverse event
3.2% (3)
4.2% (2)
2.2% (1)
Significant and
persistent skin
irritation (including
redness, itchiness,
and/or swelling)
2.2% (2)
4.2% (2)
0.0% (0)
Other: feeling of
weakness around the
wrist
1.1% (1)
2.1% (1)
0.0% (0)
Other: Stinging pain
in wrist
1.1% (1)
0.0% (0)
2.1% (1)
»
Blinding Assessment
Subjects were blinded to whether they received
stimulation or not. After the stimulation session,
subjects were asked whether they believed they
received treatment, sham, or did not know.
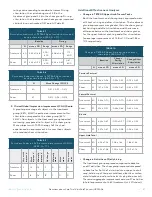
TABLE 15
Blinding Assessment (Completed Population; N = 92)
Investigational
Device
Don’t Know
Sham Device
Treatment
18
23
7
Sham
9
17
18
9.9 Study Limitations
•
The device has only been evaluated in subjects
diagnosed with Essential Tremor and the effectiveness
of the device has not been evaluated for tremor
associated with other conditions.
•
Many participants in the study were also taking
medication for their tremor and it was difficult to assess
the effect of the device compared to medication.
•
Effectiveness was only evaluated within one clinic visit
immediately after a 40 minute stimulation session.
•
The active device provided stimulation that could be
felt by the patient while the sham device was inactive.
Therefore, it is possible that some patients could have
correctly identified the treatment group they were in
which could have biased study results.