24
Questions about Cala Trio? Visit CalaTrio.com/HCPFAQs
LBL-5122 Rev C NOV 2019
9.10 Benefit/Risk Discussion
The risks of the device are based on nonclinical laboratory
studies as well as data collected in the clinical study
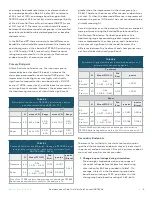
described above. Three percent
(3/93)
of enrolled
subjects experienced 4 non-serious adverse events
(one
subject reported 2 AEs.)
The 4 adverse events were mild,
anticipated, and resolved within 24 hours without any
intervention or sequelae, specifically, weakness, skin
irritation, and pain
(reported in a sham patient)
.
Should any adverse reactions occur, the Cala ONE therapy
level can be reduced
(e.g., turning down stimulation level or
reducing duration)
. In addition, the device is easily removed
from the patient’s wrist.
The probable benefits of the device are also based on
data collected in the clinical study. Subjects had an
improvement in tremor severity. In addition, subjects
had an improvement in ADLs compared to baseline.
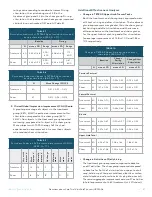
Fifty-percent of the Treatment patients had a 0.5-point
improvement in tremor severity while 27.5% had a
1.0-point improvement directly after 40 minutes of
device use. These differences are clinically meaningful.
The duration of the effect was not assessed beyond the
assessments five minutes after stimulation ended.
Additional factors to be considered in determining
probable risks and benefits for the Cala ONE include
uncertainty in: the results as a result of the lack of
statistical significance between active and sham groups
for both tremor severity and ADLs; the potential for
subjects correctly identifying their study arm due to
stimulation in the active group which may have biased
these subjects; and many participants in the study were
also taking medication for their tremor, and it was
difficult to assess the effect of the device compared to
medication. Finally, the device was only assessed during
one clinic visit. It is unknown if repeated treatment
will provide better, worse or similar benefit. However,
the available alternatives
(e.g., medications, deep brain
stimulation and focused ultrasound)
have the potential for
many adverse effects, some of which can be serious and/
or cause death. The Cala ONE device uses non-invasive
technology which has been available for many years with
a more favorable risk profile.