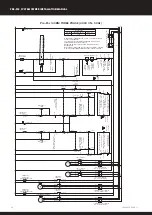
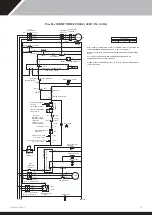
5.0 ELECTROLYTIC CORROSION IN SWIMMING POOLS
Electrolytic corrosion will occur when dissimilar metals that are in contact with
each other create a potential difference between themselves. Sometimes
separated by a conductive substance known as an electrolyte, the dissimilar
metals will create a small voltage (potential difference) that allows the ions of
one material to pass to the other.
Just like a battery, ions will pass from the most positive material to the more
negative material.
Anything more than 0.3 volts can cause the most positive material to degrade.
A swimming pool with its associated equipment can create this effect. The pool
water being an ideal electrolyte and components of the filtration circuit, heating
system, steps, lights etc providing the dissimilar metals needed to complete the
circuit.
Whilst these small voltages are rarely a safety threat, they can create premature
failure through corrosion. Not dissimilar to corrosion through oxidation,
electrolytic corrosion can cause complete failure of a metallic material in a very
short period of time.
In order to prevent this type of corrosion all metallic components in contact with
swimming pool water should be bonded together using 10mm² bonding cable.
This includes non-electrical items such as metal filters, pump strainer boxes,
heat exchangers, steps and handrails. It is highly recommended that bonding
be retrofitted to existing pools, which may not be protected by this system.
11
SD566252 ISSUE 51