GentleYAG Laser System
Candela Corporation
8501-00-1766 Revision F
CONFIDENTIAL
Page 140
0123
This product may be covered by one or more of the
following U.S. patents:
6,200,308 6,059,772 6,041,787 6,032,675 6,026,816
5,979,454 5,814.040 5,810,801 5,599,342 5,598,426
5,400,791 5,360,425 5,312,395 5,287,380 5,109,387
5, 066,293 4,887,600 4,829,262 6,171,301,B1
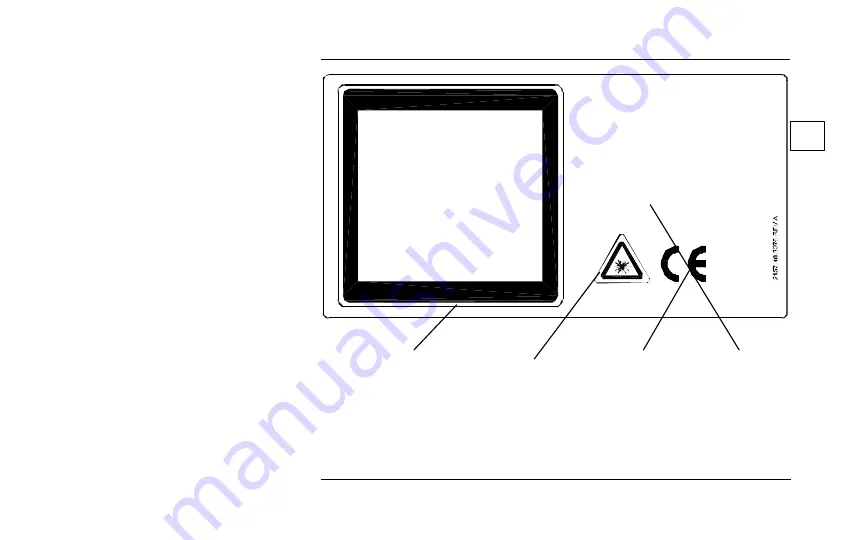
Complies with FDA performance
standards for laser products except
for deviations pursuant to Laser
Notice No. 50 dated July 26, 2001.
INVISIBLE LASER RADIAT ION
AVOID EYE OR SKIN EXPOSURE
TO DIRECT OR SCAT TERED RADIATION
CLASS 4 LASER PR ODUCT
( Per EN60825- 1: 2002-07)
MAXIMUM PULSE ENERGY 80 J
PULSE WIDTH 0.25 - 300 ms
WAVELENGTH 1064 nm
AIMIN G BEAM
CLASS 1
MAXIMUM POWER
5 mW
PULSE WIDTH
CW
WAVELENGTH
520-550 nm
10-A,B,C,D,E
Label Identification
C
B
A
E
D
Summary of Contents for GentleYAG
Page 1: ...Operator s Manual 0123 8501 00 1766 Revision F June 2006 ...
Page 4: ...GentleYAG Laser System Candela Corporation 8501 00 1766 Revision F CONFIDENTIAL Page 4 ...
Page 48: ...GentleYAG Laser System Candela Corporation 8501 00 1766 Revision F CONFIDENTIAL Page 48 ...
Page 144: ...GentleYAG Laser System Candela Corporation 8501 00 1766 Revision F CONFIDENTIAL Page 144 ...
Page 150: ...GentleYAG Laser System Candela Corporation 8501 00 1766 Revision F CONFIDENTIAL Page 150 ...
















































