User Manual: xr100+_eng
7
Cardioline S.p.A.
Via Linz,151
38121 Trento (TN)
info@cardioline.it
www.cardioline.com
Sales office:
Via F.lli Bronzetti, 8 20129 Milano (MI) Italy tel.+39 02 94750470
fax +39 02 94750471
xr100+
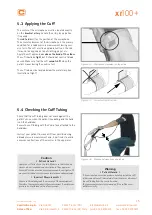
Safety Information for
Non‑Invasive Blood Pressure Measurement
Warning
• Patient Hazard •
Do not take blood pressure measurements with a cuff on
patients suffering from sickle cell anemia or if skin lesions are
likely to occur.
The cuff may cause hematomas in patients with severe blood
coagulation disease. In these instances, the user must take a
decision for or against automatic blood pressure measure
ments.
Caution
• Compromised Measuring Accuracy •
Arrhythmias occurring frequently during a measurement may
compromise the accuracy of the measurement.
Valid measurements may not be possible under certain circum
stances.
Electromagnetic fields are also capable of impairing the mea
suring accuracy.
Note
• If the cuff pressure exceeds the maximum value of
300 mmHg during inflation, the inflation procedure will
be aborted and the cuff deflated. As a redundant safety
precaution, the cuff is immediately deflated when the cuff
pressure exceeds 320 mmHg.
You can check the proper functioning of this safety pre
caution by abruptly bending your arm while the cuff is
being inflated, causing a brief overpressure in the cuff.
The cuff must deflate immediately.
• Measurements that do not yield a valid measurement will
not be repeated during the exercise test.
• If the inflation phase takes longer than 40 seconds or if an
adequate pressure does not build up in the cuff within a
reasonable period of time, the measurement will be aborted
and the cuff deflated.
• If a valid measurement cannot be completed within
120 seconds, the measurement will be aborted and the cuff
deflated.
• If the cuff pressure remains constant for some time, the
measurement will also be aborted and the cuff deflated.
Intended Use
The xr100+ is a computer-controlled medical ergometer,
which operates at pedal speeds between 30 and 130 RPM
and loads between 6 and 999 W.
The speed-independent range is shown in section 9.4 on
page 35.
The xr100+ ergometer may only be used in exercise testing
and for rehabilitation of cardiac and cardiovascular patients
according to the instructions given in this manual. If the
ergometer is used for other purposes, the manufacturer
cannot be held liable for personal injuries or property dam-
age resulting from the unintended use of the equipment.
Note – Applied Parts
• Applied parts are components that are directly in contact
with the human body (e.g., blood pressure measuring
devices).
Note – Stability
• Ensure the stability of the ergometer. If the maximum
per mitted patient weight is exceeded, the stability of the
ergometer can no longer be guaranteed. It may become
unstable as a result.
Biocompatibility
The parts of the product described in this manual, including
all accessories that come in contact with the patient during
the intended use, fulfill the biocompatibility requirements
of the applicable standards if applied as intended.
If you have questions in this matter, please contact ergoline
or a representative.
Applicable Laws, Regulations and Directives
If you have questions regarding laws, regulations or direc-
tives related to the product, please contact ergoline GmbH.