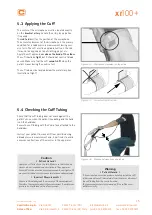
6
User Manual: xr100+_eng
Cardioline S.p.A.
Via Linz,151
38121 Trento (TN)
info@cardioline.it
www.cardioline.com
Sales office:
Via F.lli Bronzetti, 8 20129 Milano (MI) Italy tel.+39 02 94750470
fax +39 02 94750471
xr100+
2 Safety Information
Danger
• Explosion Hazard •
The device is not designed for use in areas where an explosion
hazard may occur.
Explosion hazards may result from the use of flammable anes
thetics, skin cleansing agents, or disinfectants.
Warning
• Patient Hazard, Equipment Damage •
Do not expose the xr100+ to direct sunlight to prevent system
components from reaching inadmissible high tempe ra tures.
Do NOT use the xr100+ outdoors (medical device). Furthermore
the device has no additional protection against the ingress of
humidity. Humidity inside the device may cause equipment
malfunctions and increases the risk of an electric shock.
Additionally, the device should not be operated in the vicinity
of power systems, because they may impair equipment func
tions.
The xr100+ may only be used in combination with accessories
approved by ergoline GmbH.
• Risk to Persons •
Before using the ergometer, the operator must ascertain that it
is in correct working order and operating condition. The cables
and connectors, in particular, must be checked for signs of
damage. Damaged parts must be replaced immediately.
• Equipment Malfunction •
Only the special shielded cables supplied by ergoline may be
used to connect the device to other pieces of equipment.
• Equipment Malfunction •
Cellular telephones may not be used in the immediate vicinity
of the ergometer, because they might interfere with the proper
functioning of the ergometer.
Electromagnetic interference most probably exists when the
watt reading is unstable. If the displayed value changes fre
quently even though the speed is above 30 RPM, this may be
due to electro magnetic interference.
Warning
• Shock Hazard •
When the ergometer is connected to other equipment or if a
medical system is created, it must be ensured that the added
leakage currents do not present a hazard. In case of questions,
please contact your ergoline dealer or the ergoline GmbH
Service Department.
For use, the ergometer must always be connected to electric
installations that fulfill the local requirements.
• Patient Hazard •
The German Medical Device Operator Ordinance (MPBetreibV, § 5)
demands that users
• must be trained in the use of the ergometer
• must be familiar with the routines for handling and
assembly of the ergometer
• must be familiar with and observe the safety rules and
regulations for operation of this type of equipment
• must be informed about any other pertinent rules and
regulations (e.g. safety instructions)
• must be informed about the potential hazards arising from
the use of this type of equipment.
• make sure that no unauthorised changes are carried out.
Note
Only the removal of the power cord will result in an all pole
disconnection of the device from mains.
Caution
Additional equipment connected to medical electrical equip
ment must comply with the respective IEC or ISO standards
(e.g., IEC 60950 for data processing equipment).
Furthermore, all configurations must meet the requirements of
the applicable medical systems standards (see 3rd edition of
IEC 606011).
Anybody connecting additional equipment to medical electri
cal equipment configures a medical system and is therefore
responsible that the system complies with the requirements for
medical electrical systems. Attention is drawn to the fact that
local laws take priority over the above mentioned require
ments.
If in doubt, please consult your local dealer or ergoline GmbH.