Endosafe
®
- Portable Test System User’s Guide
Copyright 2003
Page A1
PIPTS101-10
A
A
p
p
p
p
e
e
n
n
d
d
i
i
x
x
A
A
Endosafe
®
- PTS Endotoxin
PERFORMING ROUTINE ENDOTOXIN TEST
A routine PTS LAL assay is conducted by following the simple prompts on the PTS
instrument. The following represents a typical assay procedure:
1.
Instrument Operation
x
Press
the
MENU
key on the PTS keypad to turn instrument on (Menu 5 turns
instrument off).
x
The PTS Reader initiates a “SYSTEM SELF TEST” as it heats up to 37°C –
this takes approximately 5 minutes.
x
The PTS Reader displays “SELF TEST OK” and then “INSERT
CARTRIDGE”.
2.
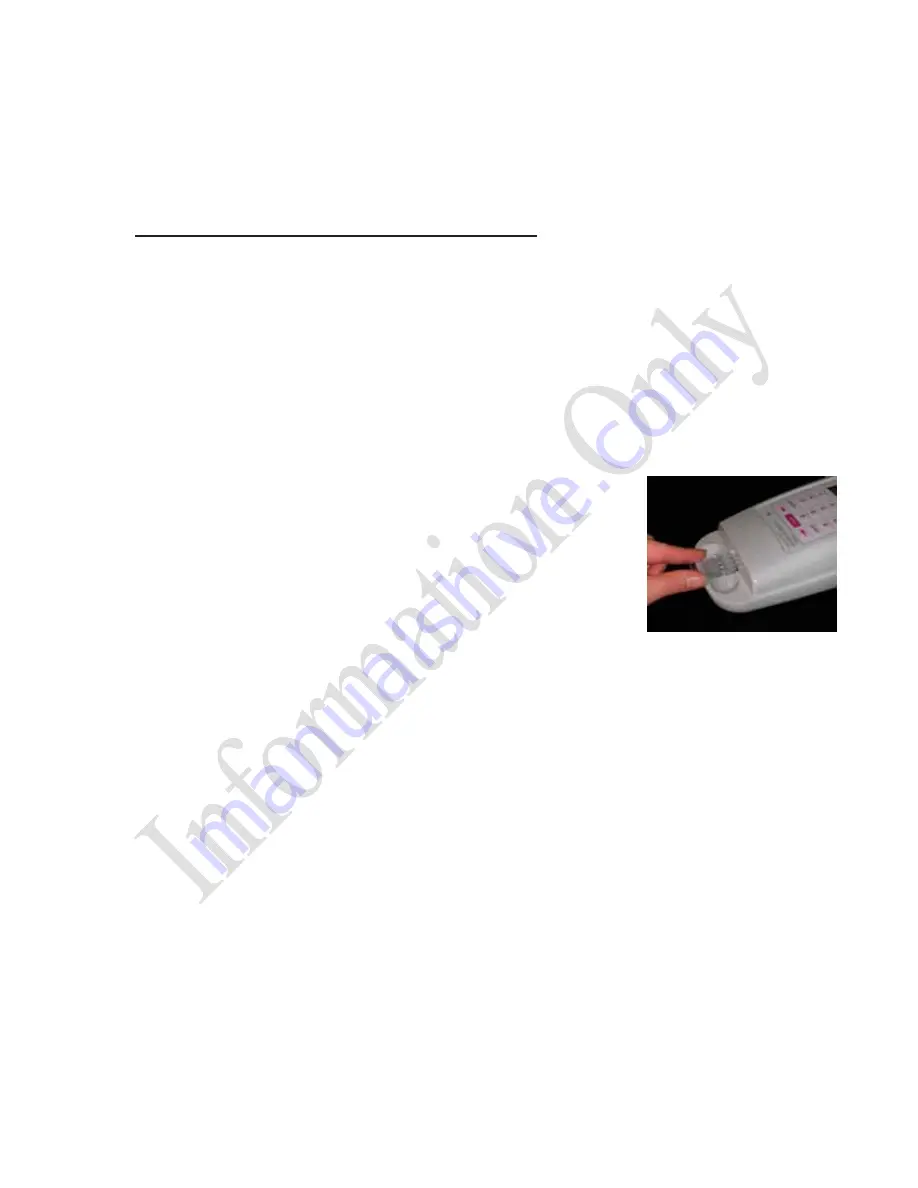
Insert the Cartridge
Note: Allow the cartridge to come to room temperature in
pouch before use.
Remove cartridge from pouch and insert with sample
reservoirs facing up into slot at front of the PTS Reader.
Press cartridge firmly into slot.
3.
Enter Required Information
Once the cartridge has been firmly inserted into the PTS Reader, the PTS
Reader prompts the user to enter the following information:
x
Enter OID
(Operator ID)
x
Enter Lot #
(Cartridge Lot #)
x
Enter Calibration Code
(If the Calibration Code for the particular lot # has
already been entered, the PTS Reader does not prompt for the code again.
(To Erase all stored lot #’s and corresponding calibration codes, select menu,
2, then 4 from the initial menu)
x
Lot #
(Confirms cartridge lot number entered)
x
Enter Sample Lot #
x
Enter Sample ID
(Selecting and scrolling with the menu key under the
sample ID Header allows for fifty (50) samples to be entered and stored)
x
Enter Dilution Factor
x
Note:
All LAL Reagent Water (LRW) used to make product dilutions should
be tested for the absence of detectable endotoxin.
While the above information is being entered into the PTS Reader, the
cartridge is being pre-warmed.