RSR-000885-000 (6)
Page
18
of
20
8.
Labeling
8.1.
Explanation of Symbols
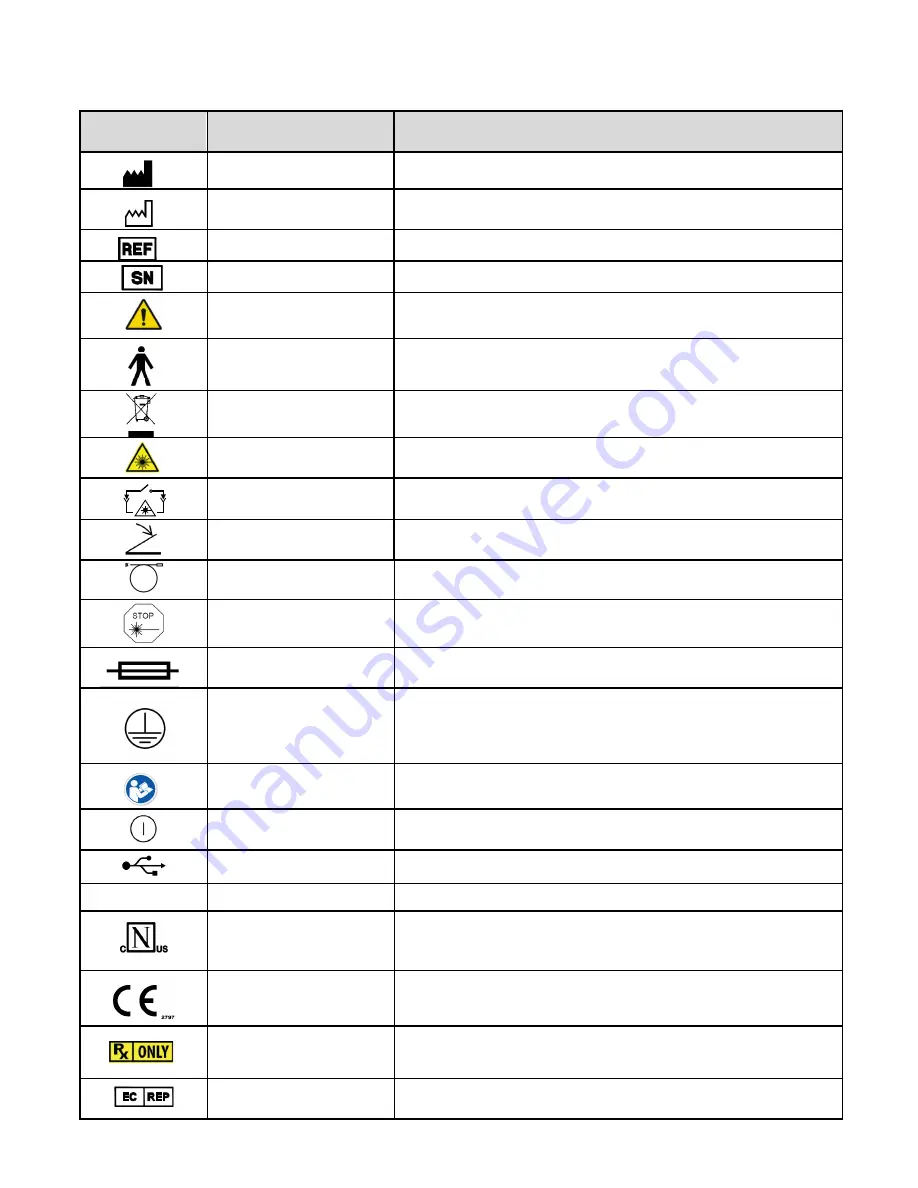
Symbol
Title
Description
Manufacturer
Indicates the medical device manufacturer, as defined in EU Directives
90/386/EEC, 93/42/EEC and 98/79/EC.
Date of manufacture
Indicates the date when the medical device was manufactured.
Catalog number
Indicates the manufacturer’s catalog, or model, number so that the medical device
can be identified.
Serial number
Indicates the manufacturer’s serial number so that a specific medical device can
be identified.
Caution
Indicates the need for the user to consult the instructions for use for important
cautionary information such as warnings and precautions that cannot, for a variety
of reasons, be presented on the medical device itself.
Type B Applied Part
Refers to the part of the medical device which comes into physical contact with the
patient in order for the device to carry out its intended function.
Do not dispose in unsorted
municipal waste (WEEE)
Equipment must not be disposed of as unsorted municipal waste.
Laser Warning
Warning label for class 2 and higher laser radiation
Remote interlock connector
Identifies remote interlock connection port
Foot Switch
Identifies connection port for foot switch
Optical fiber applicator
Identifies connection port for hand piece fiber
Emergency Laser Stop
Button used to terminate laser emission and shutdown the device in the event of
an emergency.
Fuse
To identify fuse boxes or their location.
Note:
Not user replaceable.
Protective earth (ground)
To identify any terminal which is not intended for connection to an external
conductor for protection against electrical shock in case of a fault, or the terminal
of a protective earth (ground).
Note:
This is located inside the device.
Follow Instructions
Indicates the need for the user to consult the instructions for use prior to
operating the device.
Power On/Off
Push/push power button
USB Port
Connection for software updates and Instant Replay Backup / Restore
IOIOI
VGA Port
For Manufacturer Use ONLY
60601-1
Nemko-CCL Safety Mark with
NRTL indicators
Indicates compliance with the Certification Body (Nemko-CCL) requirements
regarding Electrical Safety (60601-1) in the US and Canada
CE Marking of Conformity
Certification mark that indicates conformity with health, safety, and environmental
protection standards for products sold within the European Economic Area.
Prescription devices
CAUTION – Federal law restricts this device to sale by or on the order of a licensed
practitioner licensed by the law of the State in which the practitioner practices to
use or order the use of the device
Authorized representative in the
European Community
Indicates the Authorized representative (REP) in the European community (EC).
Summary of Contents for LIGHTFORCE XLi
Page 251: ...RSR 000885 000 6 Pagina 7 di 22 ...