RSR-000885-000 (6)
Page
19
of
20
Note:
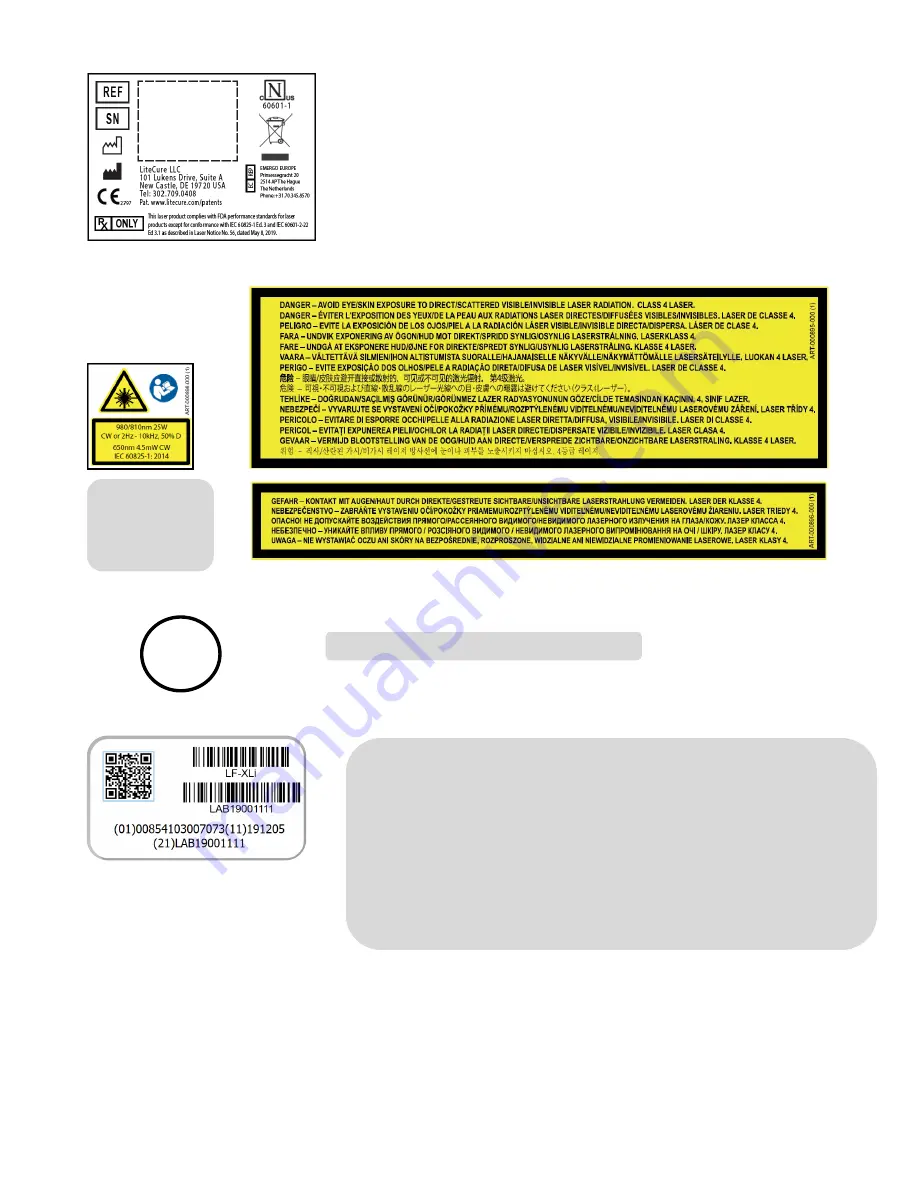
Example UDI shown for reference only. This format applies to medical, human use
laser products only. For those models, the 2D, or QR, code represents the UDI text at the
bottom of the label and each number provides information unique to the device as follows:
(01) Indicates Device Identifier number, which is a Global Trade Identification Number
(GTIN-14) that can be used to search for the device registration in the FDA GUDID
Database.
(11) Indicates Date of Manufacture in YYMMDD format.
(21) Indicates Serial Number.
The linear barcodes are for manufacturer use only.
Note:
Example
label shown
above. Refer to
side of device for
actual label.
Note:
Warranty void if seal is broken.
8.2.
Laser Product Label
8.3.
Laser Warnings
8.4.
Warranty Seal
8.5.
Unique Device Identification (UDI) Label
WARRANTY
VOID
IF SEAL
IS BROKEN
Summary of Contents for LIGHTFORCE XLi
Page 251: ...RSR 000885 000 6 Pagina 7 di 22 ...