8Max
6
4
2
8Max
6
4
2
8Max
6
4
2
8Max
6
4
2
8Max
6
4
2
8Max
6
4
2
Medication
chamber
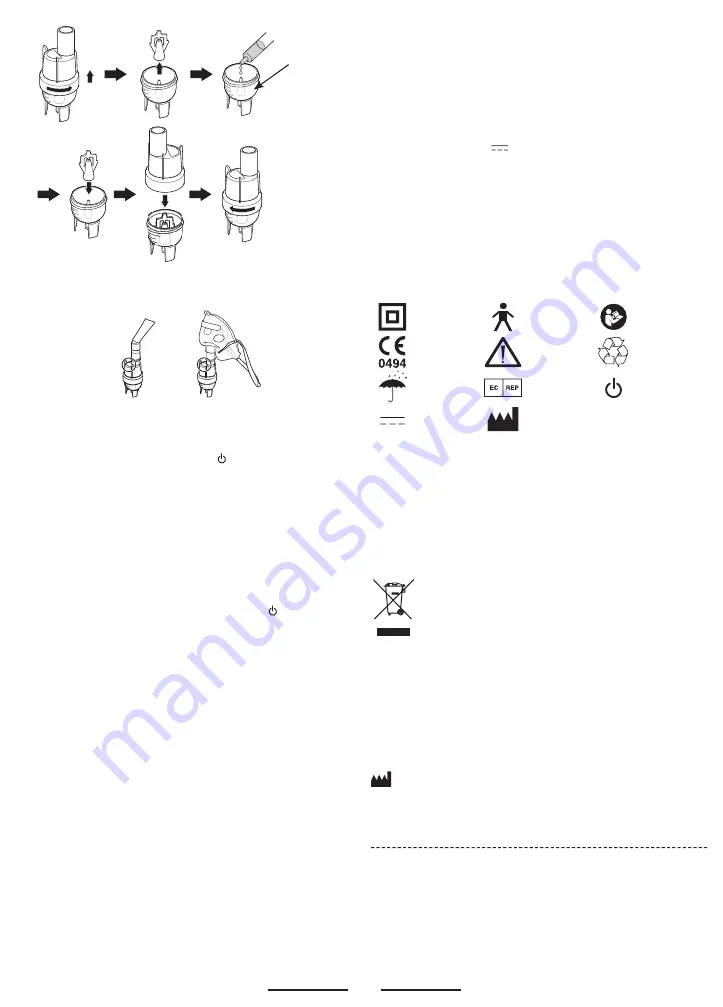
• According to the type of treatment you wish to carry out and the
method you choose, attach either the face mask or the mouthpiece
to the upper section of the medication chamber.
8max
6
4
2
8max
6
4
2
Your appliance is now ready to use.
To carry out aerosol-therapy treatment
• Plug your appliance into a socket of the correct voltage.
• Press the switch of the appliance with .
• Start the treatment and carry on for as long as you can still see the
‘mist’ produced by nebulization of the medication performed by the
appliance.
• One treatment should be used for less than 45min (equipped with
45min-timer) and after the treatment should be taken a break at
least 45 min off.
Warning:
The presence of a small amount (about 0.4 ml) of medication
in the medication chamber at the end of each treatment is absolutely
normal. This amount, called residue volume, cannot be nebulized.
At the end of each treatment
• Turn off the appliance by pressing its switch with .
• Unplug the appliance from the power socket.
• Wind up the power supply cord.
• Detach the face mask (or mouthpiece) from the medication
chamber.
• Detach the exible tubing from both the medication chamber and
the appliance’s air outlet.
• Wipe the face mask (or mouthpiece) and the medication chamber,
clean according to the instructions in the following paragraph.
CLEANING
To avoid inef cient nebulization or contamination, clean all parts after
each use, making sure to remove any residual medication.
■
Washable parts
Wash with warm water and mild detergent (neutral detergent). Rinse
each part thoroughly with clean, hot tap water and allow them to air
dry in a clean location.
■
Non-washable parts
Compressor, Air Tube
After making sure that the power plug is unplugged from the power
outlet, wipe clean with a soft cloth moistened with water or a mild
detergent (neutral detergent).
Air Filter
Do not wash or clean the lter. If the lter becomes damp or wet for
any reason, replace it immediately as it may cause air blockage.
DISINFECTING
Disinfect the parts once a week. If the parts are heavily stained, replace
them with new ones.
CHANGING THE AIR FILTER
We strongly suggest that you change the air lter within 60days, or when
the lter appears grey in color. To change the lter, you will need to:
• unscrew the cover by turning it anti-clockwise with a screw driver
and take off the cover of the compartment which houses the air lter.
• remove the old lter and insert a new one.
• replace the cover by turning it clockwise with a screw driver.
TECHNICAL SPECIFICATION
• Motor: Compact piston compressor model BD80 CX
• Power supply: 12VDC
1.25A
• Working pressure Max: >1bar=14.5PSI
• Working pressure Max: 2.0bar=29.0PSI
• Air ow: 8.0 l/min (Max.)
• Dimensions: 95(W) X 135(D) X 55(H) mm
• Weight: 430g
• Operating temperature: 10°C to 40°C (50°F to 104°F)
• Storage temperature: -40°C to 70°C (-40°F to 158°F)
• Atomizer capacity: 8ml (Max.)
• Noise level: Less than 50 dBA/1m
• Particle size: 2.44
μ
m
CLASSIFICATION
Class II
equipment
Type B
equipment
Refer to the
instruction
manual
CE mark
Caution
Recycling
Keep dry
EU
representative
Standby
Direct current
Manufacturer
WARRANTY
The legal provisions in this respect apply. The warranty is limited to
defective material and workmanship. AC adapter and cable are not
covered by the warranty. The warranty is only valid if the product has
neither been opened nor subjected to violence or willful damage and it is
return with the original receipt. Contact your dealer if you have any com-
plaints. For further information, contact the customers service (E-mail:
sales-oe@systems.citizen.co.jp). In the event of warranty claims being
deemed to be justi ed, the customer concerned will be supplied with a
replacement product. The customer is only entitled to receive a com-
parable replacement product. CITIZEN cannot be held liable for any
consequential damage. Subject to error and changes.
ATTENTION – Instructions for a correct disposal of the prod-
uct
This symbol indicates that the product must not be
disposed with domestic waste after its usage, but in
accordance with the legal provisions in force, in order to
avoid negative health and environmental implications.
Notes:
• The device ful lls the provisions of EC Directive 93/42/ECC
(Medical Device Directive) and European Standard EN13544-
1:2007+A1:2009, Respiratory therapy equipment- Part1: Nebulizing
systems and their components.
• The device may not operate properly if the temperature and voltage
conditions differ from those stated in the speci cations.
• Performance may vary with drugs such as those in suspension
or high viscosity form. Refer to your drug supplier’s data sheet for
further information
BREMED LIMITED
Unit 1104, 11/F., Two Harbourfront, 22 Tak
Fung Street, Kowloon, Hong Kong
Eng 1
Eng 2
CITIZEN is a registered trade mark of Citizen Holdings Co., Japan
Design and Speci cation are subject to change without notice.
Ver. 1401
CITIZEN SYSTEMS JAPAN CO., LTD.
6-1-12, Tanashi-cho, Nishi-Tokyo-shi,
Tokyo 188-8511, Japan
E-mail: sales-oe@systems.citizen.co.jp
http://www.citizen-systems.co.jp/













