27
DS/EN 62304
Medical device software – software life cycle processes
DS/EN ISO 14971
Application of risk management to medical devices
EN 301 489-17 V3.1.1
Electromagnetic Compatibility (EMC) standard for radio equipment
and services; Part 17
EN 300 328 V2.1.1
Wideband transmission systems; Data transmission equipment
operating in the 2,4 GHz ISM band
6.3 Declaration of conformity
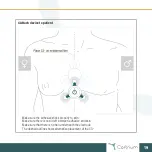
The C3
+
is in conformity with the essential requirements and provisions of the EU Medical Device
Directive (MDD).
2
2 COUNCIL DIRECTIVE 93/42/EEC of 14 June 1993 concerning medical devices.