MHW 0002 – DK 1693 | Version 001 – 14/09/2017 | custo med GmbH
2
[2] Resting and Stress ECG hardware, custo cardio 200 |
page 18
Resting and Stress ECG
Hardware, description of device for custo cardio 200
2.9
Manufacturer's declaration regarding EMC (electromagnetic compatibility)
according to DIN EN 60601-1-2:2007
Lead lengths
Patient leads
approx. 1450 mm (extremity leads),
approx. 1200 mm (chest wall leads)
USB cable
approx. 5000 mm
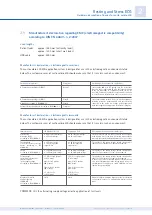
Manufacturer's declaration – electromagnetic emissions
The custo cardio 200 ECG application system is designed for use in the electromagnetic environment stated
below. The customer or user of custo cardio 200 should make sure that it is used in such an environment.
Manufacturer’s declaration – electromagnetic immunity
The custo cardio 200 ECG application system is designed for use in the electromagnetic environment stated
below. The customer or user of custo cardio 200 should make sure that it is used in such an environment.
COMMENT: U
T
is the alternating supply voltage prior to application of test levels
Emission measurements
HF emissions according to CISPR11
HF emissions according to CISPR11
Harmonics according to IEC61000-3-2
Voltage fluctuations/flickers according to IEC61000-3-3
Compliance
Group 1
Class B
Class A
Complies
Immunity tests
Electrostatic
discharge (ESD)
according to IEC 61000-4-2
Fast transient
electric interference factors/
bursts according to IEC
61000-4-4
Surges
according to IEC 61000-4-5
Voltage drops,
short-time interruptions
and fluctuations
in the supply voltage
according to IEC 61000-4-11
Magnetic field with
supply frequency
(50/60 Hz)
according to IEC 61000-4-8
IEC 60601 test level
± 6 kV contact discharge
± 8 kV air discharge
± 2 kV for net wires
± 1 kV for input and
output wires
± 1 kV push-pull voltage
± 2 kV push-push voltage
< 5 % U
T
for 0.5 periods
(> 95 % drop)
40 % U
T
for 5 periods
(60 % drop)
70 % U
T
for 25 periods
(30 % drop)
< 5 % U
T
for 5 s
(> 95 % drop)
3 A/m
Compliance level
± 6 kV contact discharge
± 8 kV air discharge
± 2 kV for net wires
± 1 kV for input and
output wires
± 1 kV push-pull voltage
± 2 kV push-push voltage
< 5 % U
T
for 0.5 periods
(> 95 % drop)
40 % U
T
for 5 periods
(60 % drop)
70 % U
T
for 25 periods
(30 % drop)
< 5 % U
T
for 5 s
(> 95 % drop)
3 A/m
Electromagnetic environment - guidelines
custo cardio 200 uses HF energy only for its internal
function. custo cardio 200 BT uses the frequency band
in the 2.4 GHz range to communicate with the PC. Its
level of HF emission is therefore very low and is unlikely
to be sufficient to interfere with other electronic devices.
custo cardio 200 is suitable for use in all establishments,
including domestic establishments and those directly
connected to the public low voltage power supply net-
work that supplies buildings used for domestic purposes.
Electromagnetic environment - guidelines
Floors should be made of wood or concrete or be equipped
with ceramic tiles. If the floor is provided with synthetic
material, the relative air humidity must be at least 30 %.
The quality of the supply voltage should correspond to
that of a typical business or clinical environment.
The quality of the supply voltage should correspond to
that of a typical business or clinical environment.
The quality of the supply voltage should correspond to
that of a typical business or clinical environment.
If the user of custo cardio 200 requires continued func-
tion, even if interruptions in the energy supply occur, it is
recommended to supply custo cardio 200 from an inter-
ruption-free power supply.
Magnetic fields with mains frequency should correspond
to the typical values of a business and clinical environ-
ment.