6
ABPM with custo screen 300 and custo diagnostic | GEB 0154 – DK 1055 | Version 002 – 05.06.2013 | custo med GmbH
Introduction
01
01.1
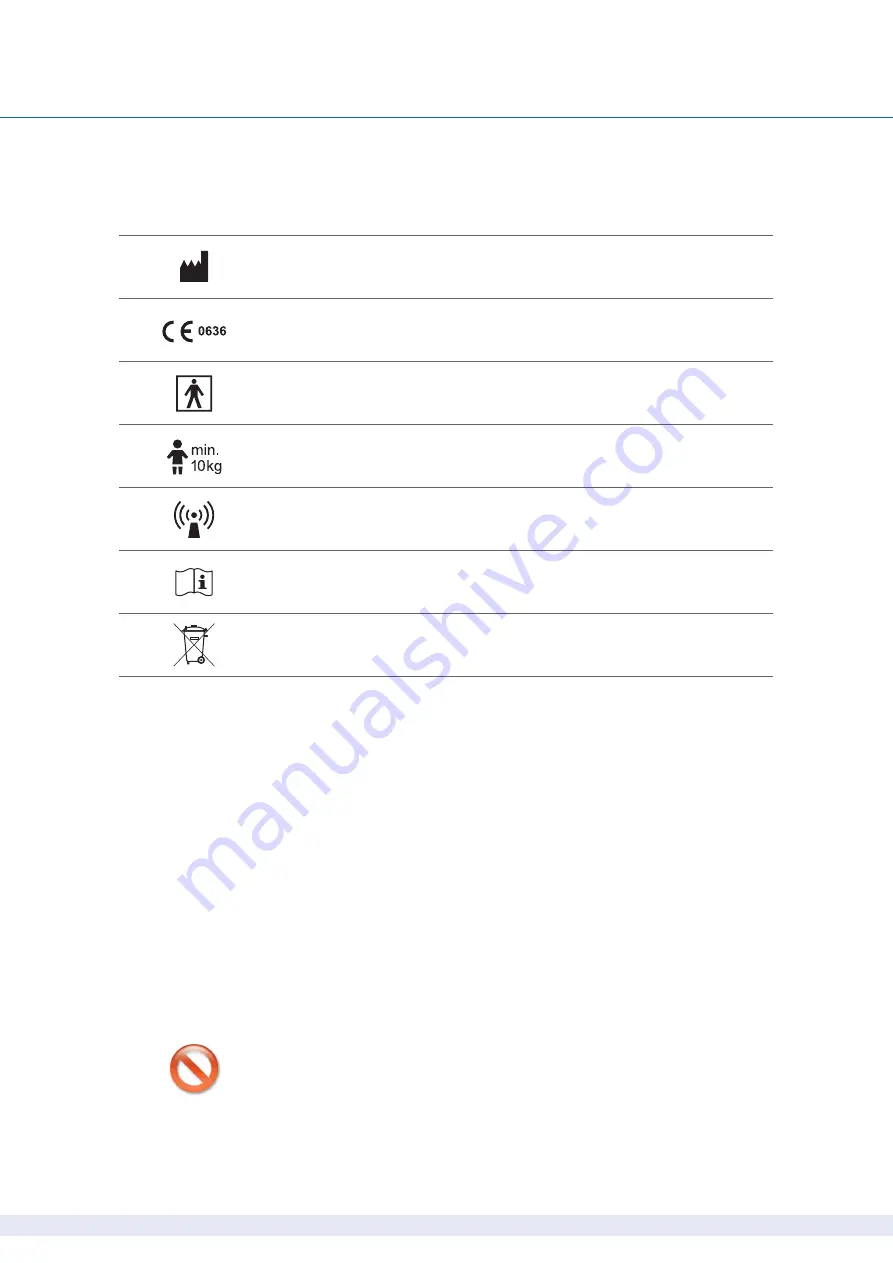
Symbols on the devices
Manufacturer:
custo med GmbH, Leibnizstr. 7, 85521 Ottobrunn, Germany
CE mark
Protection class classification of medical electrical
equipment according to IEC 60601-1 (Type BF)
The device is not suited for small children or newborns
Non-ionizing electromagnetic radiation,
device has a HF transmitter (radio unit not active for custo screen 300)
Observe the Operating Manual
Separate collection of electrical and electronic equipment,
do not dispose with domestic waste
01.2
Intended
use
custo screen 300 is an ABPM recorder with an internal power supply which is
used to record and evaluate the blood pressure behaviour of a patient. The
recording period can last for up to 72 hours. custo screen 300 is perfectly safe
for use by patients with a pacemaker.
The system is intended for use by trained specialists or physicians in clinics and
medical practices. Patients are only allowed to use the recording device after
receiving instruction by trained specialists. Patients who are not capable of un-
derstanding and following the instructions given are not allowed to use the
device. This applies in particular to senile patients or patients suffering from
dementia.
The device is not suited for unsupervised use
with unconscious patients.
Summary of Contents for custo screen 300
Page 2: ......







































