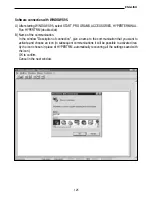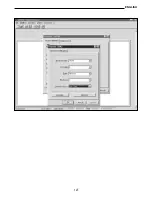
If a temperature probe is connected to input B, the instrument will use the temperature value
measured at input B for the dissolved Oxygen calculations and not the temperature measu-
red by the sensor inside the Oxygen probe. The user must consider this carefully so as not
to make incorrect measurements.
Notes on the operation of the polarographic probe.
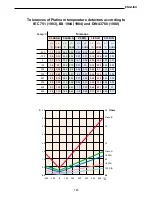
In polarographic probes the dissolved Oxygen measurement is taken by measuring a current pro-
portional to the partial pressure of the Oxygen present in the liquid, generated by a chemical reac-
tion which takes place inside the probe.
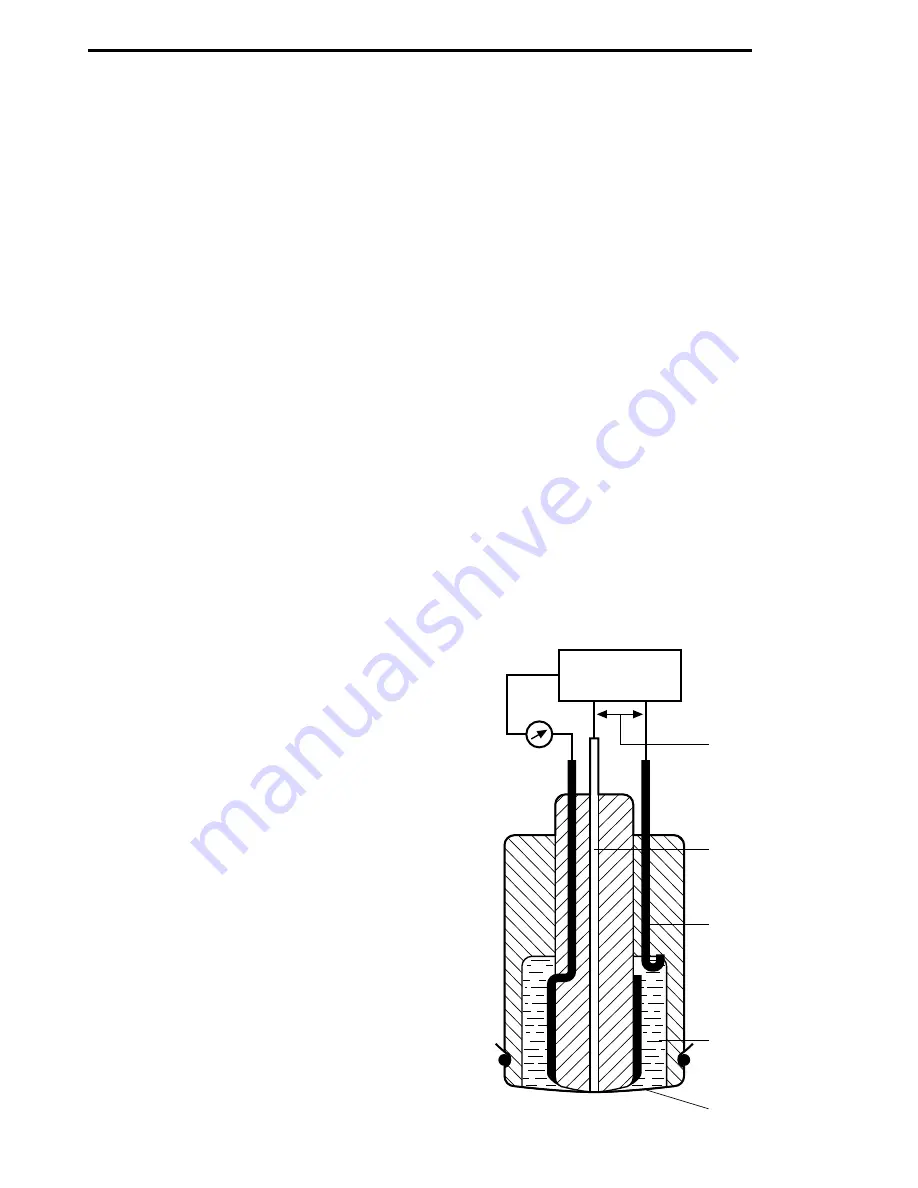
The probe is composed of an external membrane of PTFE or FEP (10÷50 µm) which is oxygen-
permeable and which contains the electrolytic solution used in the measurement,.
The membrane is in direct contact with the cathode of the probe in which an oxygen reduction reac-
tion takes place which is spread through the membrane, with consequent consumption of electrons.
The position of the membrane is such as to isolate the cathode from the internal electrolytic solu-
tion and from the other electrons, guaranteeing the flow of electrons only through the measuring
circuit. Inside the probe there are two other electrodes: the reference electrode, used for polarising
the activation of the measurement system with a suitable voltage, so as to trigger the redox reac-
tion, and the anode in which the oxidation reaction takes place with the liberation of electrons. The
measurement and internal regulation circuit of the instrument rebalances the potential between the
anode and the cathode, measuring the generated current, which will be proportional to the quantity
of reduced Oxygen in the cathode and therefore in the liquid being examined.
The oxidation reaction in the anode uses up the
salt in the electrolytic solution contained in the
probe; for this reason the electrode is exhausted
and must be recalibrated from time to time. If salt
consumption is high, the electrolytic solution in
the probe must be replaced. If the surface of the
electrodes is filled with scale produced by the
redox reaction, the electrodes must be chemical-
ly cleaned, completely regenerating the sensor.
There are two types of probes on the market
which require no maintenance and which, once
exhausted, must be replaced.
On the market there are also dissolved Oxygen
probes with two electrodes: the anode and the
reference electrode are combined in a single
electrode, which supplies the polarisation volta-
ge necessary to establish the redox reaction and
allows the passage of current necessary to
restore equilibrium between the cathode and the
anode; the temperature sensor inside the probe
allows the measurement of the temperature of
the liquid being examined. With this type of pro-
ENGLISH
138
I
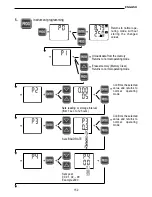
Potentiostat
Working electrode
(cathode)
Reference
electrode
Electrolyte
Membrane
Polarisation
voltage