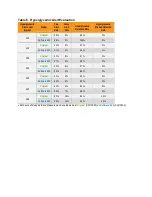
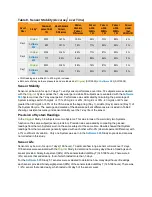
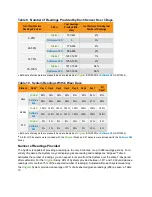
Subjects in both studies used the System for seven days. In the
Original
Study, thirty-six subjects each
wore 2 sensors; in the
Software 505
Study, all subjects wore 1 sensor only. Throughout the 7-day wear
period, the sensor was calibrated with an average of 2 fingersticks per day (approximately once every 12
hours). In the
Original
Study, subjects used the LifeScan
®
OneTouch
®
Ultra
®
2 meter and in the
Software
505
Study, subjects used Bayer's CONTOUR® NEXT USB meter.
In the
Original
Study, all subjects were evaluated in a controlled clinic environment on all three clinic
days: Day 1, Day 4, and Day 7 of the 7-day wear period. In the
Software 505
Study, subjects were
evaluated in one of the three clinic days so there are fewer data samples than in the
Original
Study.
While using the System in the clinic, subjects had their blood glucose measured every 15 minutes with a
reliable laboratory method, the Yellow Springs Instrument 2300 STAT Plus
™
Glucose Analyzer. This
instrument is referred to as the “YSI.” Readings from the System were reported every 5 minutes and
paired with YSI values in order to characterize how well the System readings agreed with laboratory
standard blood glucose results. The remainder of the study took place at home, and the System
performance was also paired with the comparative meter results, referred to as the “SMBG.”