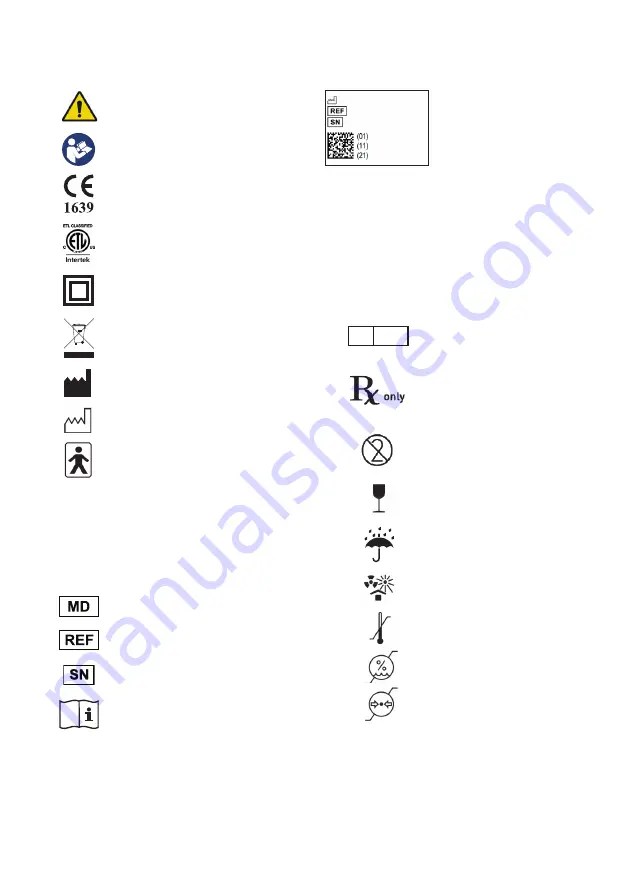
Example of a UDI label
This is a statement that alerts the
user to the possibility of serious
injury or other adverse reactions
with the use or misuse of the device
This is a statement that alerts the
user to the possibility of a problem
with the system associated with its
use or misuse
Authorised Representative in the
European Community
Caution: Federal (USA) law restricts
this device to sale on or by the
order of a licensed healthcare
professional
Single use only - do not reuse
Fragile, handle with care
Keep dry
Protect from heat and radioactive
sources
Temperature limitation
Humidity limitation
Atmospheric pressure limitation
Warning
Refer to instruction manual
/ booklet
Medical Devices Directive 93/42/EEC
Medical Device Regulation 2017/745
North America ETL listed
Class II Equipment (Double Insulated)
Do not dispose of with the normal
household waste
Manufacturer
Date of Manufacture
Suitable for connection to type BF applied
parts
IP: Ingress Protection
2: Protection against fingers or other
object not greater than 80mm in length
and 12mm in diameter
2: Protection from vertically dripping water
when tilted to 15
o
)
Medical Device
Catalogue number
Serial number
Operating Instructions
12345
1234567890
YYYYMMDD
0 5060178 XXXXX X
YYMMDD
1234567890
IP22
WARNING
CAUTION
1. Explanation of Label Symbols and Statements
2
V E N T U R I
®
C O M PA C T
D H G - H E A LT H C A R E . C O M
EC REP
Note: Abbreviation:
Negative Pressure Wound Therapy
is abbreviated to ‘
NPWT
’ throughout this document.





































