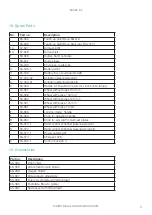
BURE XL
DIRECTHEALTHCAREGROUP.COM
8
13. Safety Instructions
The Bure Walker may only be used:
• As a WALKING AID, indoors
• On HARD, LEVEL surfaces
Max. user weight: 240 kg
14. Recycling/Disposal
When the product is no longer usable it should be recycled in accordance with legislation and regulations in
the country concerned. All electrical parts including batteries must be removed and recycled as electrical
components. (Ask your local recycling station for further information about how the different
materials in the product (metals, plastics, electronics) should be recycled).
15. Warranty
We provide two years warranty against manufacturing faults (does not apply to wear parts). For further
information or enquiries please contact:
Direct Healthcare Group Sverige AB
Torshamnsgatan 35,
SE-164 40 Kista, Sweden
Tel: +46 (0)8-557 62 200
info.se@directhealthcaregroup.com
www.directhealthcaregroup.com