3
PURPOSE OF THIS DEVICE
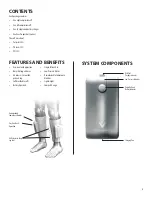
The purpose of the VenaPro is to aid in the prevention of Deep Vein Thrombosis (DVT) by helping to stimulate blood flow in the legs. This is accomplished by an electronically controlled pump
delivering a set amount of air to the leg cuffs that, in turn, compress the calf or calves to aid blood flow out of the lower extremities.
The pump will inflate each leg cuff to a preset pressure of 50 mmHg and deflate once the pressure is reached. The cycles are repeated on each unit until the power is turned off. Internal
rechargeable batteries allow the VenaPro to be completely portable, thus preventing interruptions in treatment.
User Profile:
The intended users are the patient, caretaker or a family member providing assistance. The user should be able to:
•
read and understand the operator’s manual, warnings and cautions
•
manually place the compression cuff on the body part to be treated
•
sense auditory and visual signals
Indications for Use:
The VenaPro Vascular Therapy System is intended to be an easy to use portable system, prescribed by a physician, for use in the home or clinical setting to help prevent the onset of DVT in
patients by stimulating blood flow in the extremities (simulating muscle contractions).
This device can be used to:
•
Aid in the prevention of DVT
•
Enhance blood circulation
•
Diminish post-operative pain and swelling
•
Reduce wound healing time
•
Aid in the treatment of: stasis dermatitis, venous stasis ulcers, arterial and diabetic leg ulcers, chronic venous insufficiency and reduction of edema in the lower limbs
•
As prophylaxis for Deep Vein Thrombosis (DVT) by persons expecting to be stationary for long periods of time
CONTRAINDICATIONS
The VenaPro MUST NOT be used to treat the following conditions:
Persons with suspected, active or untreated: deep vein thrombosis, ischemic vascular disease, severe arteriosclerosis, pulmonary edema, severe congestive heart failure, thrombophlebitis, or an
active infection.
On the legs where cuffs would interfere with the following conditions: vein ligation, gangrene, dermatitis, open wounds, a recent skin graft, massive edema or extreme deformity of the leg.
On any neuropathy.
On extremities that are insensitive to pain.
Where increased venous or lymphatic return is undesirable.
WARNINGS
•
The VenaPro cuffs are designed for single patient use only.
•
Device is to be used only by the patient prescribed, and only for its intended use.
•
Operation of this device can be done by the patient.
•
To avoid tripping or falling, do not walk with cuffs on your legs while the device is charging.
•
Keep this device out of the reach of children and away from household pets and pests.
•
The VenaPro is a standalone device that uses a DJO AC Adapter and Battery Charger only (see Using the AC Adapter and Battery Charger section) and is not to be used or
interconnected to any other device.
•
Do not open or remove covers. No user serviceable parts inside. Direct all unit issues to your local Customer Service representative.
•
If you experience pain, swelling, sensation changes or any unusual reactions (including allergic reactions to the materials used in this device) while using this device, stop
using this device and consult your medical professional immediately.
•
If pulsations or throbbing occur, the cuff may be wrapped too tightly. Loosen Immediately.