QBC STAR System Operator’s Manual
6000-300-000 5–6
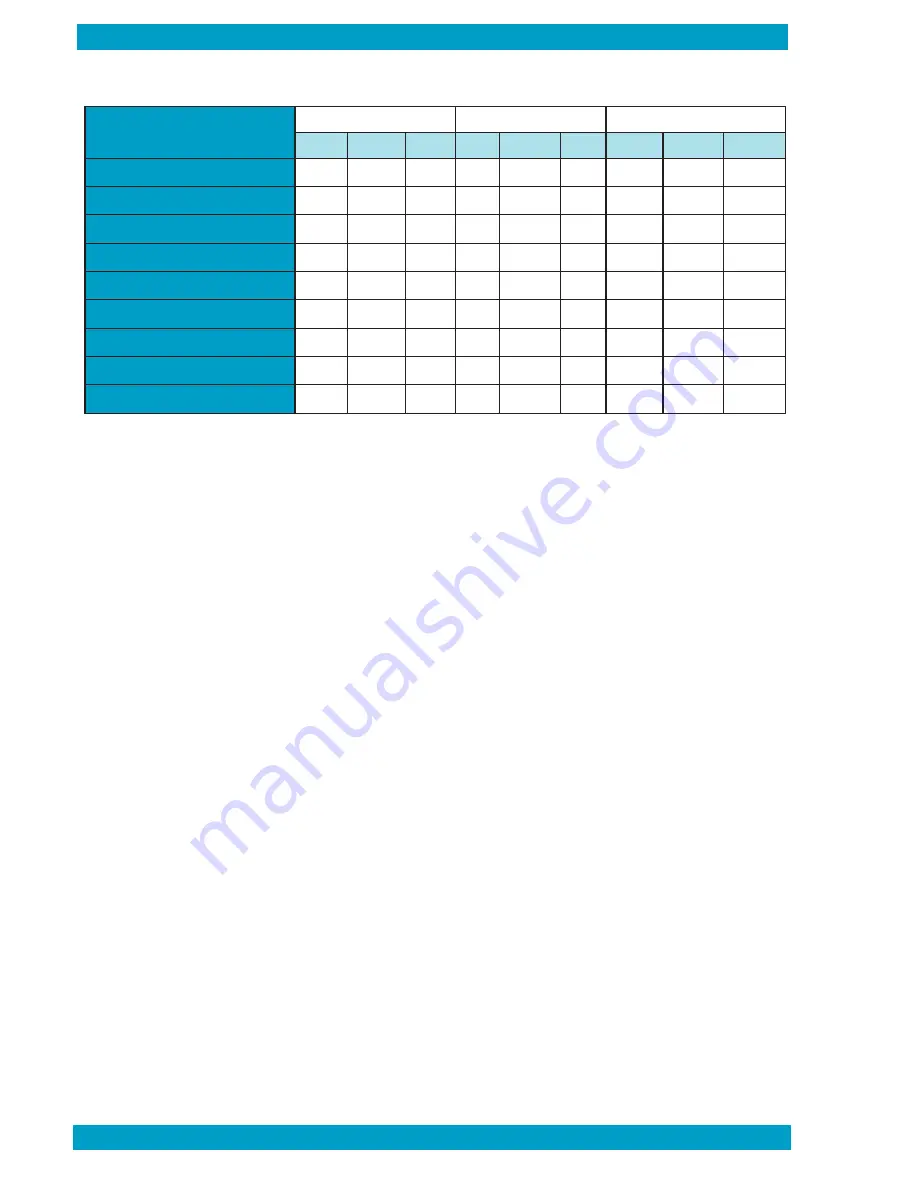
Level 1
Level 2
Level 3
Min
Target
Max
Min
Target
Max
Min
Target
Max
HCT (%)
36.9
37.9
38.9
44.9
45.9
46.9
65.1
66.1
67.1
HGB (g/dL)
12.3
12.9
13.5
14.8
15.6
16.4
20.6
21.7
22.8
MCHC (g/dL)
31.6
34.0
36.6
31.6
34.0
36.5
30.7
32.8
35.0
PLT (x 10
9
/L)
90
100
110
342
360
378
615
647
679
WBC (x 10
9
/L)
4.6
5.7
6.8
9.5
10.6
11.7
48.5
53.5
58.5
GRAN (x 10
9
/L)
2.2
2.7
3.2
6.0
6.5
7.0
28.3
31.3
34.3
%GRAN
38
47
57
56
61
67
54
59
63
L/M (x 10
9
/L)
2.4
3.0
3.6
3.5
4.1
4.7
20.2
22.2
24.2
%L/M
43
53
62
33
39
44
37
41
46
6000-300-000 5-6
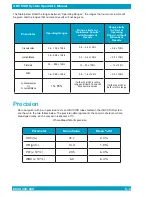
The QC label tolerances are shown in the table below.
Analytical Quality Control
The QBC STAR Centrifugal Hematology System has multiple built-in analytical quality control (QC)
systems that maintain the overall system integrity and the quality of the test results it produces.
The QBC STAR System has five analytical quality control elements:
1. Factory calibration. System calibration is set during manufacture and cannot be altered by
the user.
2. Instrument Power On Self-Test. This test assures that each time the instrument is turned
on, the computer, memory, optics, and motors are fully functional. Should you choose to
leave the system on continuously; the test will automatically be repeated every 8 hours if
the door is closed. A tri-level quality control label (QC label), designed to simulate 3 hema-
tology specimens (simulating low cell counts, normal cell counts, and elevated cell counts)
tests the system’s optics against values established at the time of manufacture. At the end
of the power on self-test, the instrument prints the values obtained from reading the QC
label. The values may be plotted to evaluate for shifts or trends in the data. The instrument
will flag any results that are outside the set limits, print an error code, and automatically
shut down operation of the instrument until the problem is corrected and a valid power on
self-test is performed.
3. Electronic QC (during each sample run). The built-in electronic checks during each sample
run confirm the proper centrifuge speed, centrifugation profile, system communications,
and internal temperature.
4. Sample Preparation QC (during each sample run). The built in checks confirm that the QBC
STAR tube has not been previously processed. Tests confirm that the tube assembly is
the proper length, the float is present and the correct length, and the tube is filled with the
correct amount of blood.
5. Reagent QC (during each sample run). These built-in checks evaluate sample and reagent
integrity using the data from the optical scan. This includes tests for fluorescent signal
intensity, proper number, size and location of the cell layers and interface sharpness.
When these analytical quality control checks are successfully completed, the status of the instru-
ment’s analytical QC is printed on the patient record as “STAR Analytical QC: Passed.”Results are
reported only if all of the analytical quality control requirements have been satisfied.
Summary of Contents for QBC STAR
Page 14: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Page 28: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Page 40: ...QBC STAR System Operator s Manual 6000 300 000 4 12 THIS PAGE INTENTIONALLY LEFT BLANK...
Page 50: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Page 72: ...6000 300 000 A 1 8 Appendices 6000 300 000 8 1...
Page 74: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Page 76: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Page 78: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Page 80: ...THIS PAGE INTENTIONALLY LEFT BLANK...