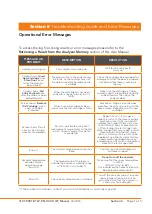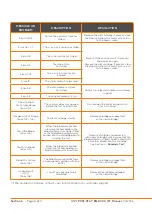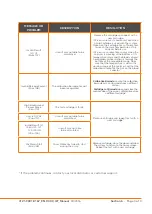
Section 3 -
Page 4 of 6
3121-9001-0167_EN.03.00_QT_Manual
04/2016
Calibration
The Quo-Test A1C System is certified by the NGSP and IFCC.
The Quo-Test Analyzer and A1C Test Cartridges have been calibrated using samples
provided by the European Reference Laboratory via the NGSP network.
Results obtained using the Quo-Test A1C System are traceable to the IFCC reference
method.
Reported Units
The Quo-Test A1C assay reports results in up to two user selectable units: % DCCT,
mmol/mol IFCC, % JDS, eAG mg/dl or eAG mmol/l.
mmol/mol IFCC = (% DCCT - 2.15) x 10.929
% JDS = (0.09274 x mmol/mol IFCC) + 1.724
eAG mg/dl = (28.7 x % DCCT) – 46.7
eAG mmol/l = (1.59 x % DCCT) – 2.59
eAG values are based on a correlation study linking % DCCT to the patient’s average
glucose concentration, resulting in published formula to derive the eAG.
eAG values may differ significantly from a patients glucose level measured at the
same time.
The Quo-Test Analyzer allows the user to select dual reporting (two different
measument units can be displayed) or single reporting. Users should refer to national
guidance when setting up reported units.
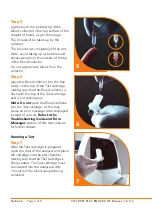
% DCCT is the default primary unit, to change the primary reported unit press
Change
Primary Units
DCCT
OK
Change
Summary of Contents for Quo-Test
Page 1: ...User Manual Quo Test Diagnostics for life ...
Page 32: ...3121 9001 0167 ...