Section 2 -
Page 1 of 3
3121-9001-0167_EN.03.00_QT_Manual
04/2016
General Information
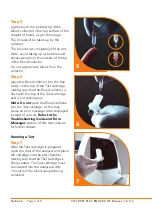
Please read these instructions in full before performing a test
The Quo-Test Analyzer is for
in vitro
diagnostic use only.
The Quo-Test Analyzer is for use at the point of care.
Only Quo-Test Cartridges can be used with the Quo-Test Analyzer.
Unpacking
Unpack the system and check that the following components are present in the pack.
If there is anything missing contact your local distributor or customer support.
• Quo-Test Analyzer
• Barcode Scanner
• Mains Power Cable
• Power Supply
• User Manual
It is recommended to keep the packaging to transport your analyzer. If the optional
printer has been purchased, it will be delivered in a separate box.
Warnings and Precautions
• There are no user serviceable parts inside the analyzer. Call your local distributor or
customer support if you have any problems that are not resolved by following the
Troubleshooting Guide and Error Messages
section of this User Manual.
• Place the system on a clean, dry, flat and level surface away from direct sunlight
in a room with a temperature range of 18 to 30 °C (64 to 86 °F). An analyzer which
has been stored at a temperature above or below the working temperature will take
longer to exit the Analyzer Warm Up screen when the system is first powered on.
• Place the system away from draughts, or other sources that may cause sudden
changes in temperature e.g. air flow from air conditioning units.
• The system must be within easy reach of a mains supply outlet with a protective
earth connection.
• The system has been designed and tested to CISPR 11 Class A. In a domestic
environment it may cause radio interference, in which case you may need to take
measures to mitigate the interference.
• Ensure that the analyzer and cartridges have reached room temperature before
using the system.
• The analyzer must be used in accordance with the instructions stated in this User
Manual.
• Used cartridges must be treated and disposed of as clinical waste.
Section 2
Introduction
IVD
Summary of Contents for Quo-Test
Page 1: ...User Manual Quo Test Diagnostics for life ...
Page 32: ...3121 9001 0167 ...