Duplication prohibited
MIR 9000
Environnement
S.A
2–3
JULY 2009
2.1
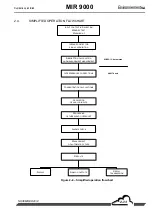
INFRARED CORRELATION PRINCIPLE
All polyatomic gases absorb an electromagnetic radiation of a given wavelength. The quality and
quantity analysis based on this phenomenon is called absorption spectroscopy.
The MIR 9000 is a measuring device using infrared spectroscopy and correlation.
This measurement is based on a principle that is completely physical, as are almost all our current
analyzers.
2.1.1
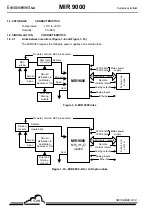
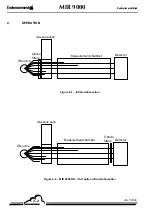
INFRARED ABSORPTION PRINCIPLE (FIGURE 2–1 AND FIGURE 2–2)
An optical ray, emitted by the IR source, passes through the measurement chamber and is focused on
an IR detector.
Each gas that is present on the path of the optical ray, absorbs the latter at defined wavelengths that
are specific to it.
An interference filter that defines a specific wavelength area is positioned on the optical path above
the measurement chamber.
Let Io be the energy received by the detector, when there is no gas in the measurement chamber, and
I the energy received by the detector when gas is present. According to the Beer Lambert law, the
following equation is obtained:
=
⇒
=
−
−
Io
I
Ln
kL
C
e
Io
I
kLC
1
1
in which:
−
k: is a coefficient depending on the gas and the wavelength,
−
L: is the length of the optical path,
−
C: is the concentration.
Since there is not just one wavelength behind an interference filter, but several wavelengths, the
equation becomes:
C f
I
Io
=
in which:
−
if is a function, depending on the type of interference filter and on the gas.
This function, f, is always close to a logarithm and can be linearized very precisely by a polynomial.
It is very difficult to measure I and Io simultaneously, for Io is the energy received by the detector
when there is no gas. Due to correlation by a gaseous filter, this problem can be avoided.
2.1.2
PRINCIPLE OF CORRELATION BY A GASEOUS FILTER
A cell filled with highly concentrated gas that needs to be measured and a cell filled with nitrogen,
which does not absorb any wavelength, are positioned on the optical path alternately.
The highly concentrated gas, which is in the cell called the reference cell, absorbs all wavelengths that
are specific to it. Even if gas molecules are present in the measurement chamber and on the optical
path, all wavelengths that should have been absorbed by them, have already been absorbed.
Therefore, the energy received by the detector can be considered to be constant and can serve as an
I
R
reference for this gas, in spite of its dependence on other phenomena such as the power of the
infrared source, the presence of particles and their opacity, etc..
Summary of Contents for Envea MIR 9000
Page 10: ...Duplication prohibited MIR 9000 EnvironnementS A 0 10 JULY 2009 Page intentionally left blank...
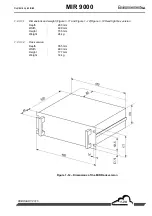
Page 22: ...EnvironnementS A MIR 9000 Duplication prohibited 1 12 JULY 2009 Figure 1 7 Analysis cabinet...
Page 27: ...Duplication prohibited MIR 9000 EnvironnementS A 1 17 JULY 2009 Page intentionally left blank...
Page 35: ...Duplication prohibited MIR 9000 EnvironnementS A 2 5 JULY 2009 Page intentionally left blank...
Page 102: ...EnvironnementS A MIR 9000 Duplication prohibited 4 2 JULY 2009 Page intentionality left blank...
Page 157: ......
Page 159: ......
Page 161: ......
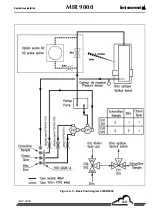
Page 163: ...EnvironnementS A MIR 9000 Duplication prohibited 6 10 JULY 2009 Figure 6 4 Oxygen probe...
Page 165: ...EnvironnementS A MIR 9000 Duplication prohibited 6 12 JULY 2009 Page intentionally left blank...
Page 180: ...EnvironnementS A MIR 9000 Duplication prohibited 6 22 JULY 2009 Page intentionally left blank...
Page 182: ...EnvironnementS A MIR 9000 Duplication prohibited 7 2 JULY 2009 Page left intentionally blank...
Page 206: ...EnvironnementS A MIR 9000 Duplication prohibited 7 28 JULY 2009 Page left intentionally blank...
Page 212: ...EnvironnementS A DAC8 board Duplication prohibited MARCH 2018 6 Page intentionally left blank...