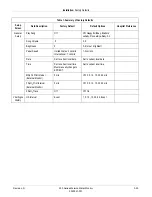
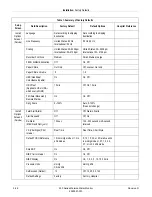
Revision D
250 Series Maternal/Fetal Monitor
4-7
2020551-001
Maintenance:
Cleaning
1. Dampen a cloth or paper towel with one of the following products; then wring
out until only slightly wet:
Sodium Hypochlorite 5.25% (Bleach) diluted 10:1
Cidex*
Sporicidin* Soap and water
2. Rub soiled area until clean.
3. Dry with a soft, dry cloth.
Maternal NIBP Cuffs and Hoses
General
The cuff must be thoroughly cleaned with the specified detergent before reuse. The
additional use of household bleach as described below provides at least
intermediate-level disinfection.
Apply cuff hose plugs before cleaning.
The following cleansing procedure was repeated 20 times on DURA-CUF
®
Blood Pressure Cuffs and once on SOFT-CUF
®
Blood Pressure Cuffs
without affecting the performance of the cuff.
While this procedure is adequate for cleaning/disinfection, it may not
remove all stains.
Do
not
immerse hoses.
Do
not
immerse cuffs without prior application of cuff hose caps.
Materials
Enzymatic detergent such as ENZOL* enzymatic detergent (US) or
Cidezyme* enzymatic detergent (UK)
Distilled water
10% solution of household bleach (5.25% sodium hypochlorite) in distilled
water
Soft cloths and soft-bristled brushes
Spray bottles
Procedure
1. Prepare the enzymatic detergent according to the manufacturer’s instructions
and the 10% bleach solution, in separate spray bottles.
2. Spray the detergent liberally on device. If the material is dried on, allow the cuff
to sit for 1 minute. For soil on the soft part of the closure or the cuff itself, wipe
the material off with a soft cloth. For persistent contamination on the soft part of
the closure, use a soft-bristled brush to loosen particles. Rinse with copious
amounts of distilled water. Repeat until no visible contamination remains. For
soil on the hook part of the closure, use a soft-bristled brush to remove the
material, and rinse with copious amounts of distilled water. Repeat until no
visible contamination remains.
*Trademarked
Summary of Contents for Corometrics 250 Series
Page 2: ......
Page 6: ...CE CE ii 0086 ...
Page 14: ...viii 250 Series Maternal Fetal Monitor Revision D 2020551 001 ...
Page 16: ...1 2 250 Series Maternal Fetal Monitor Revision D 2020551 001 For your notes ...
Page 29: ...Revision D 250 Series Maternal Fetal Monitor 2 1 2020551 001 2 Equipment Overview ...
Page 30: ...2 2 250 Series Maternal Fetal Monitor Revision D 2020551 001 For your notes ...
Page 55: ...3 1 250 Series Maternal Fetal Monitor Revision D 2020551 001 3 Installation ...
Page 56: ...3 2 250 Series Maternal Fetal Monitor Revision D 2020551 001 For your notes ...
Page 81: ...Revision D 250 Series Maternal Fetal Monitor 4 1 2020551 001 4 Maintenance ...
Page 82: ...4 2 250 Series Maternal Fetal Monitor Revision D 2020551 001 For your notes ...
Page 143: ...Revision D 250 Series Maternal Fetal Monitor 5 1 2020551 001 5 Troubleshooting ...
Page 144: ...5 2 250 Series Maternal Fetal Monitor Revision D 2020551 001 For your notes ...
Page 196: ...6 2 250 Series Maternal Fetal Monitor Revision D 2020551 001 For your notes ...
Page 239: ...Revision D 250 Series Maternal Fetal Monitor B 1 2020551 001 B Alarms Summary ...
Page 240: ...B 2 250 Series Maternal Fetal Monitor Revision D 2020551 001 Alarms Summary For your notes ...
Page 243: ...Revision D 250 Series Maternal Fetal Monitor C 1 2020551 001 C Electromagnetic Compatibility ...
Page 251: ......