1 Introduction
1.2 Regulatory information
8
Electrophoresis Power Supply EPS 3501 XL
Operating Instructions
28-9639-69
AA
International standards
CE marking
The CE marking and the corresponding Declaration of Conformity is valid for the
instrument when it is:
•
used as a stand-alone unit, or
•
connected to other CE-marked instruments, or
•
connected to other products recommended or described in the user
documentation, and
•
used in the same state as it was delivered from GE Healthcare, except for
alterations described in the user documentation or explicitly authorized by
GE Healthcare.
Regulatory compliance of
connected equipment
Any equipment connected to EPS 3501 XL should meet the safety requirements of EN
61010-1/IEC61010-1 or relevant harmonized standards. Within the European Union,
connected equipment must be CE-marked.
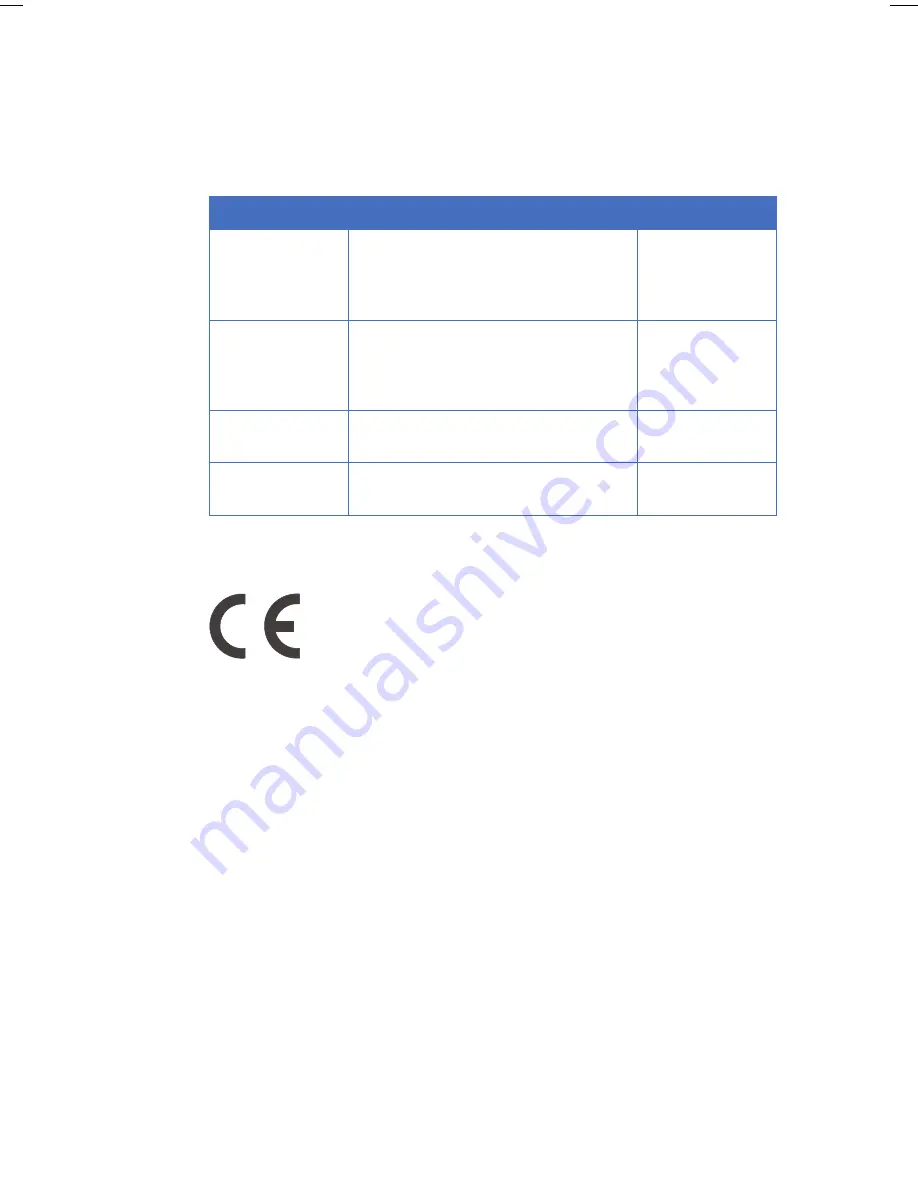
Standard
Description
Notes
EN 61010-1,
IEC 61010-1,
CAN/CSA-C22.2
No. 61010-1
Safety requirements for electrical
equipment for measurement, control
and laboratory use
EN 61326-1
EMC emissions and immunity
requirements for electrical equipment
measurement, control and laboratory
use
Harmonized with
2004/108/EC
EN-ISO 12100-1,
12100-2
Safety of machinery – Basic concepts,
general principles and design
Harmonized with
2006/42/EC
EN-ISO 14121-1,
14121-2
Safety of machinery – Principles of risk
assessment
Harmonized with
2006/42/EC









































