English (GB)
3
1.4 Responsibilities of the operator
The owner of the building or operator of the VGS-141 / VGS-143 /
VGS-145 is responsible for the following:
• Consider this manual to be part of the product and ensure that
it is kept clearly accessible in the immediate vicinity of the
system for the entire service life of the system.
• Meet the installation requirements specified by the
manufacturer (required water connections and fittings,
environmental conditions, electrical connection, protective
tube for dosing line if necessary, audible or optical warning
device for alarm messages if necessary).
• Ensure that water lines and fixings are regularly checked,
serviced and maintained.
• Obtain official approval for storing chemicals, if necessary.
• Train users in the operation of the system.
• Ensure that the regulations for the prevention of accidents are
observed in the installation location (German GUV-V D05
regulation for the prevention of accidents, "Chlorination of
Water" dated January 1997).
• Provide all users and service personnel with protective
clothing in accordance with GUV-V D05 (face mask, gloves,
protective apron).
1.5 Maintenance and service personnel
The system may only be maintained and serviced by authorised
service personnel from Grundfos.
1.6 Correct usage
The Grundfos VGS-141, VGS-143, VGS-145 may be used for
dosing chlorine (Cl
2
) as being described in this manual.
1.7 Inappropriate usage
Applications other than those listed in section
are considered not to be in accordance with the intended use and
are not permitted. The manufacturer, Grundfos, accepts no
liability for any damage resulting from incorrect use.
The system comprises state-of-the-art components and has
undergone safety-related testing.
2. Handling chlorine
2.1 Physical and chemical data
Under normal conditions of pressure and temperature, chlorine is
a yellowish green gas with a pungent odour. It exists as diatomic
molecule Cl
2
.
It is not flammable, but can under certain circumstances promote
the flammability of metals, hydrocarbons etc.
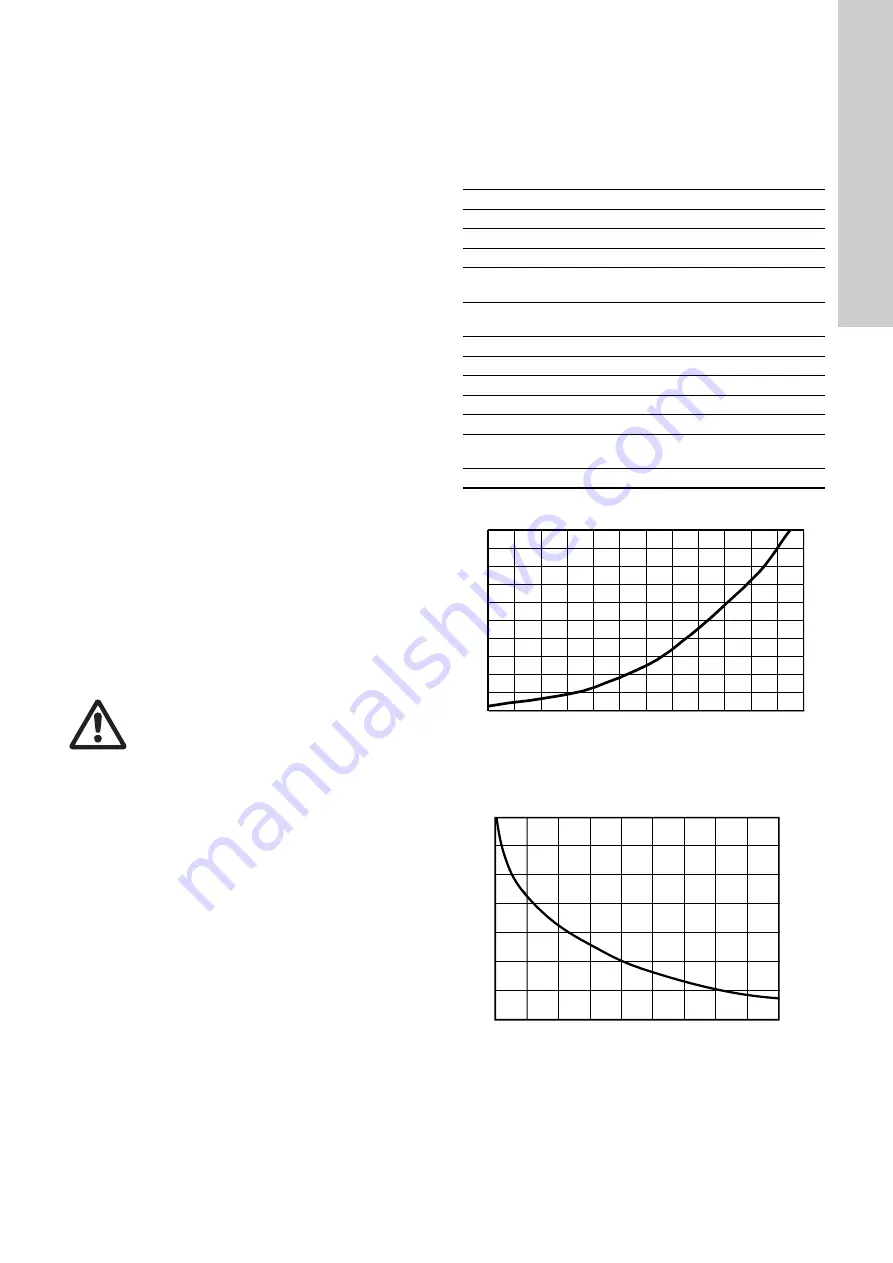
Vapour pressure curve of chlorine
Fig. 1
Vapour pressure curve of chlorine
Solubility of chlorine gas in water
Fig. 2
Solubility of chlorine gas in water
Warning
Unauthorised structural modifications to the system
may result in serious damage to equipment and
personal injury.
It is forbidden to open, modify, change the structure
of, bridge, remove, bypass or disable components,
especially safety equipment.
Atomic weight
35,457
Molecular weight Cl
2
70,941
Density (liquid)
1,57 g/cm
3
at -34,05 °C
Density (gaseous)
3,214 g/l at 0 °C, 1 bar
1 l liquid chlorine at 0 °C
corresponds to 457 l (0,457 m
3
)
gaseous chlorine
1 kg liquid chlorine at 0 °C
corresponds to 311 l (0,311 m
3
)
gaseous chlorine
Specific gravity
2,486 (specific gravity of air: 1)
Boiling point
- 34,05 °C (1 bar)
Melting point
- 100,98 °C
Evaporation heat
269 kJ/kg (at 0 °C)
Heat conductivity
0,527 kJ/m
2
h (liquid chlorine)
Degree of purity acc. to
DIN 19607
99,5 %
TLV (Threshold Limit Value)
1,5 mg/m
3
(0,5 Vol.-ppm)
TM0
4
069
1
0
9
0
8
TM
04
0
692
09
08
0
2
4
6
8
10
12
14
16
18
20
-50 -40 -30 -20 -10
0
10
20 30 40
50
60 70
Temperature (°C)
0
20
40
60
80
10
30
50
70
90
2
4
6
8
10
12
14
0
Temperature (°C)




































