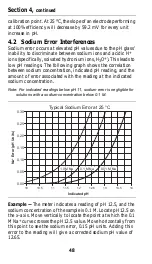
36
pH, Low Ionic Strength (LIS) Water,
continued
4.
Turn on the meter
by pressing
I/O
. Press
PH MV
until the
display shows
pH
.
5.
Press
SETUP
. Press
the up arrow three
times. Press
ENTER
to
toggle to the number
of desired decimal
places, then
EXIT
to
leave setup.
6.
In three 50-mL
beakers or cups,
prepare buffers of 4.0,
7.0, and 10.0 pH.
Note: The sample pH
should fall within the
range of the calibration
buffers.
Note: pH 6.86 buffer
may be used instead of
pH 7.0. Autobuffer
recognition for either pH
6.86 or 7.0 is user
selectable in the
sens
ion
meter setup
function.
pH mV