48
Section 4,
continued
calibration point. At 25 °C, the slope of an electrode performing
at 100% efficiency will decrease by 59.2 mV for every unit
increase in pH.
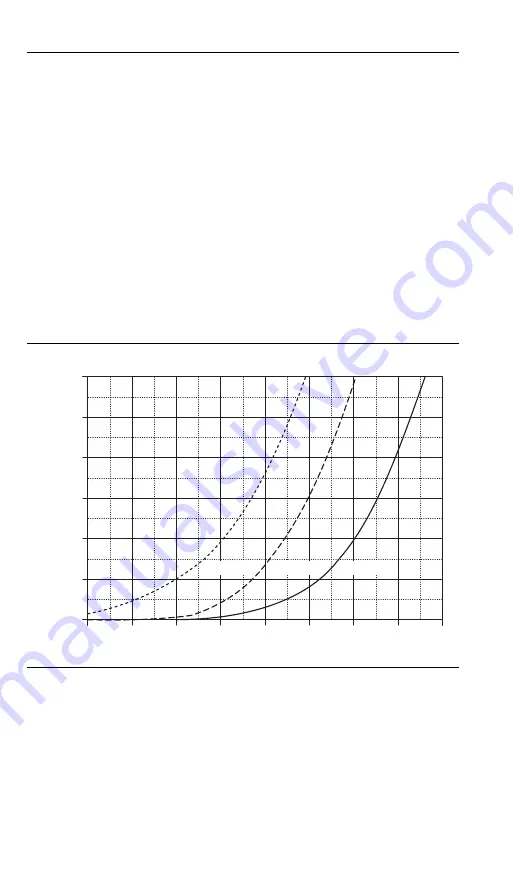
4.2 Sodium Error Interferences
Sodium error occurs at elevated pH values due to the pH glass’
inability to discriminate between sodium ions and acidic H
+
ions (specifically, solvated hydronium ions, H
3
O
+
). This leads to
low pH readings. The following graph shows the correlation
between sodium concentration, indicated pH reading, and the
amount of error associated with the reading at the indicated
sodium concentration.
Note: For indicated readings below pH 11, sodium error is negligible for
solutions with a sodium concentration below 0.1 M.
Example — The meter indicates a reading of pH 12.5, and the
sodium concentration of the sample is 0.1 M. Locate pH 12.5 on
the x-axis. Move vertically to locate the point at which the 0.1
M Na
+
curve crosses the pH 12.5 value. Move horizontally from
this point to see the sodium error, 0.15 pH units. Adding this
error to the reading will give a corrected sodium pH value of
12.65.
0.00
0.05
0.10
0.15
0.20
0.25
0.30
10
10.5
11
11.5
12
12.5
13
13.5
14
Indicated pH
Err
or (pH Units)
Na
+
Typical Sodium Error at 25 °C
1.0 M Na
+
0.1 M Na
+
0.01 M Na
+