SUMMAR
Y OF CLINIC
AL S
TUD
Y IN MEN
7
SUMMARY OF CLINICAL STUDY IN MEN
Clinical studies were conducted at multiple locations in the United States. Participants in the study were
males diagnosed with Androgenetic Alopecia, which includes hereditary hair loss, and had light to brown
skin tones. Subjects were divided into two groups: HairMax Group and a Control Group. The Control
Device looked and sounded like the HairMax device but did not include a laser light. The Clinical studies adhered
to all GCP (Good Clinical Practice) guidelines, were approved by an IRB (Institutional Review Board) and listed on
www.clinicaltrials.gov.
During the 6-month clinical study, subjects treated their hair once
per treatment, 3 times per week on non-consecutive days. The
number of thick, normal, healthy hairs in the target zone were
counted at week 1, week 16 and week 26.
Results at the 26 week visit showed that over 90% of the men
saw an increase in hair counts (based on a minimum of 32 new
hairs per square inch being observed at the follow up visit).
No subjects in the study experienced any serious adverse events.
In fact, the number and types of adverse events were similar in
the HairMax and Control Groups.
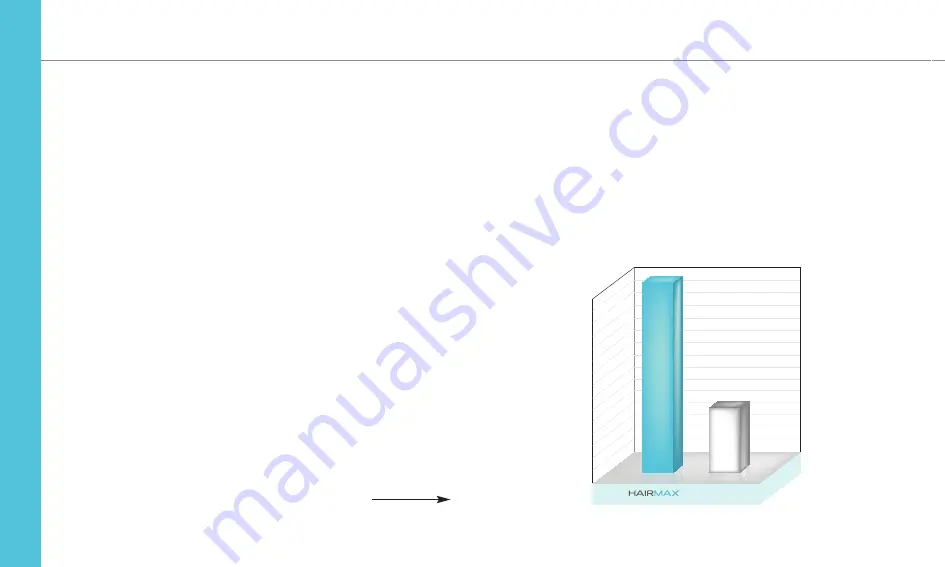
Hair Count Change
In the clinical study, after 26 weeks of treatment with the HairMax
devices versus the Control Devices, the following increases in hair
count were found.
0
10
20
30
40
50
60
70
80
90
100
110
120
130
140
0
10
20
30
40
50
60
70
80
90
100
110
120
130
140
27.7
Hairs/in2
Mean Terminal Hair Count Changes
From Baseline in
MALES
26 Weeks
Control Device
139.3
Hairs/in
2
27.7
Hairs/in
2










































