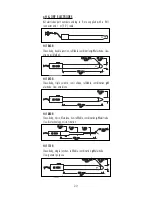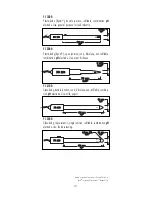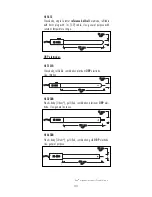
2 3
High concentrations of sodium ions interfere with readings in alkaline
solutions. The pH at which the interference starts to be significant
depends upon the composition of the glass. This interference is the
alkaline error and causes the pH to be underestimated. Hanna's
glass formulations have the indicated characteristics.
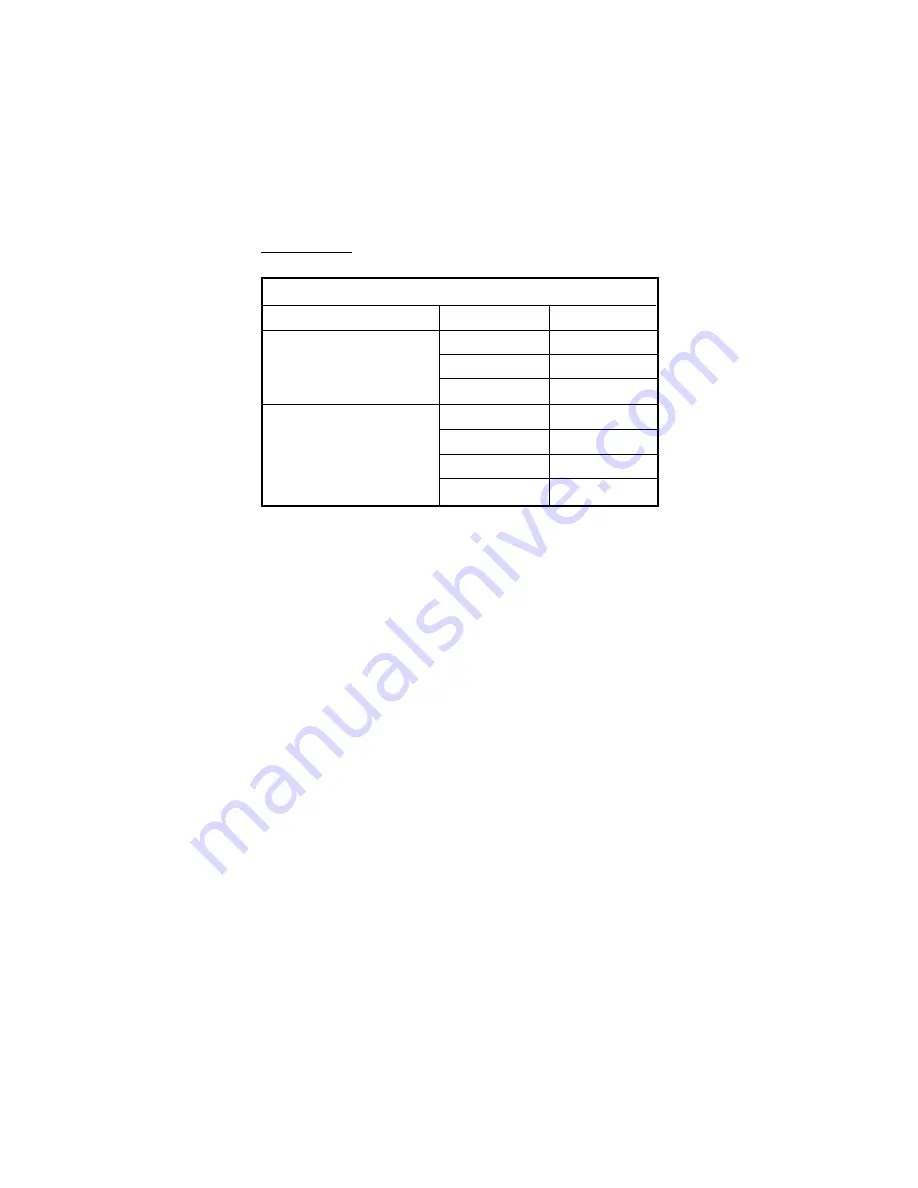
Sodium Ion Correction for the Glass at 20-25°C
Concentration
pH
Error
0.1 Mol L
-1
Na
+
13.00
0.10
13.50
0.14
14.00
0.20
1.0 Mol L
-1
Na
+
12.50
0.10
13.00
0.18
13.50
0.29
14.00
0.40
Alkaline Error