MAN-03939-001 Rev. 004 page 6 of 13
LABORATORY AND PATIENT CHARACTERISTICS
The cytology laboratories participating in the study were comprised of one referral center
(designated as S1), one screening/referral center (designated as S2) and one screening
center (designated as S3).
The screening center in the study served patient populations (screening populations) with
rates of abnormality (Low-grade Squamous Intraepithelial Lesion [LSIL] and more severe
lesions) similar to the United States average of less than 5%.
3
The referral center in the
study served a high risk referral patient population (referral populations) characterized by
high rates (>10%) of cervical abnormality. The screening/referral center’s abnormality rate
was a combination of the two previously mentioned rates. Table
1 describes the
laboratories and the patient populations.
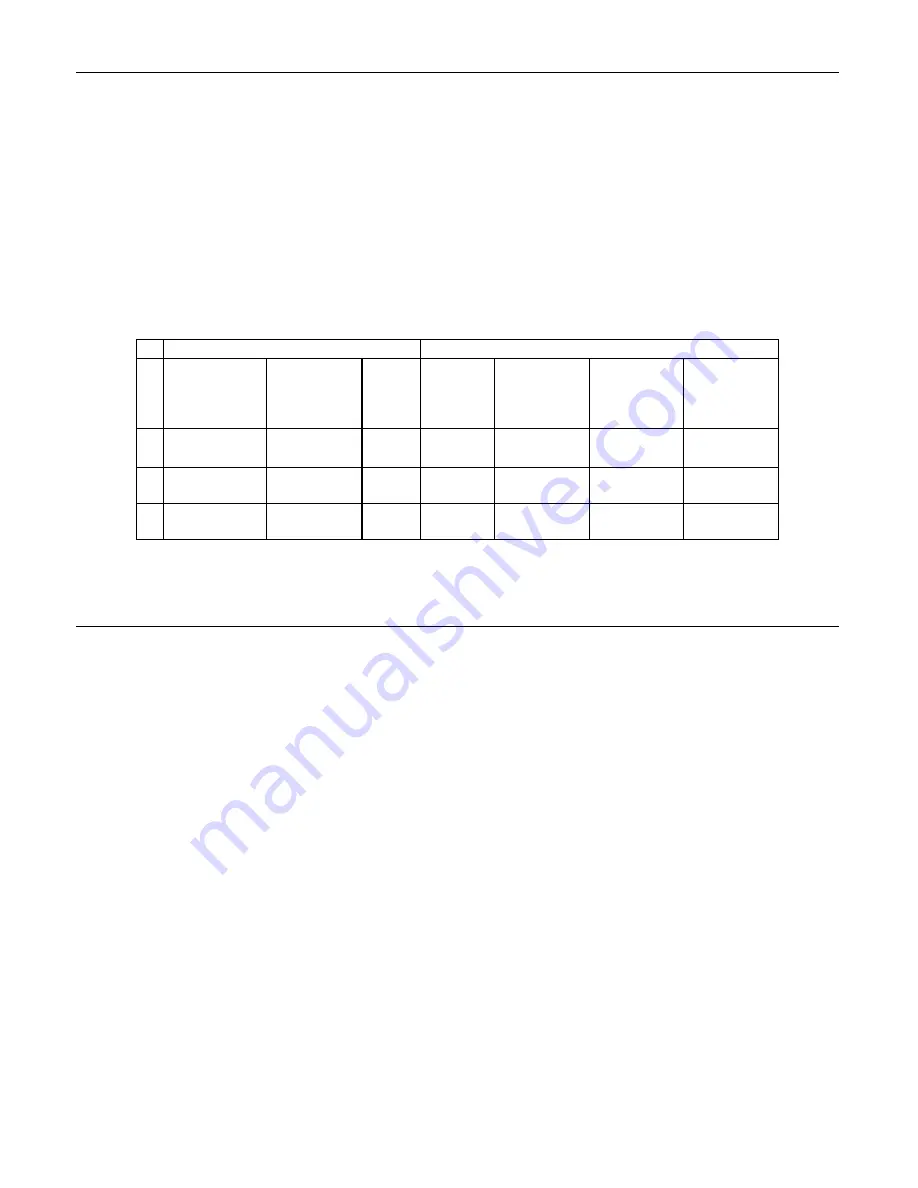
Table 1: Site Characteristics
Laboratory Characteristics
Clinical Study Demographics
Site Type of Patient
Population
Laboratory
Volume -
Smears per
Year
Cases Patient
Age Range
Post
Menopausal
%
Previous
Abnormal
Pap Smear
%
Con-current
Infection
%
S1 Referral
44,709 1188 18-85
11.8
51.8
35.2
S2
Screening/Refer
ral
62,195 1141
18-77
6.0
21.8
15.1
S3 Screening
90,639
1198 18-82
12.5
22.7
10.2
Cases with patient’s age less than 18 years or patients with a hysterectomy were
excluded from this analysis.
CLINICAL STUDY RESULTS
The diagnostic classes of the Bethesda System are used to present the comparison between
the TP-3000 and TP-2000 findings from all of the clinical trial sites.
Three independent pathologists served as an adjudication panel for the three clinical sites.
The panel reviewed all discordant cases (a one-grade or higher cytologic difference) for
descriptive diagnosis and specimen adequacy. Since a true reference cannot be determined
in such studies and therefore true sensitivity cannot be calculated, the use of an independent
adjudicated review provides an alternative to histologic confirmation by biopsy or human
papillomavirus (HPV) testing as a means for determining the reference diagnosis.
Consensus was determined when a minimum of 2 out of 3 independent pathologists
rendered an equivalent diagnosis. If a majority vote could not be obtained, a consensus was
achieved during a review by all three pathologists at a multi-headed scope.
Table 2 shows the unadjudicated descriptive diagnosis results from all sites for the TP-
3000 and TP-2000. Of the 3,527 total patients enrolled in the study, 3,224 were included
in the descriptive diagnosis analysis after all data integrity sorting was applied.
Few cases of cervical cancer were represented in the clinical study, as is typical in the
United States patient population.
4
Summary of Contents for ThinPrep 3000
Page 1: ......
Page 4: ...The ThinPrep Processor The ThinPrep Processor ...
Page 5: ...MAN 03939 001 Rev 004 page 1 of 13 Instructions for Use ...
Page 18: ...Table of Contents Table of Contents ...
Page 23: ...1 Introduction 1 Introduction ...
Page 42: ...2 ThinPrep 3000 Installation 2 ThinPrep 3000 Installation ...
Page 55: ...3 PreservCyt and CellFyx Solutions 3 PreservCyt and CellFyx Solutions ...
Page 71: ...4 Sample Collection and Preparation 4 Sample Collection and Preparation ...
Page 80: ...5 Instrument Operation 5 Instrument Operation ...
Page 105: ...6 Maintenance 6 Maintenance ...
Page 148: ...7 Troubleshooting 7 Troubleshooting ...
Page 205: ...8 Staining and Coverslipping 8 Staining and Coverslipping ...
Page 212: ...9 The ThinPrep Pap Test Training Program 9 The ThinPrep Pap Test Training Program ...
Page 215: ...10 User Interface Screens 10 User Interface Screens ...
Page 226: ...Index Index ...
Page 232: ...INDEX Index 6 ThinPrep 3000 Processor Operator s Manual This page intentionally left blank ...
Page 233: ...Service Information Service Information ...
Page 236: ...Ordering Information Ordering Information ...
Page 243: ...Safety Data Sheets Safety Data Sheets ...
Page 246: ...Appendix Appendix ...
Page 255: ......
Page 256: ......











































