15
16
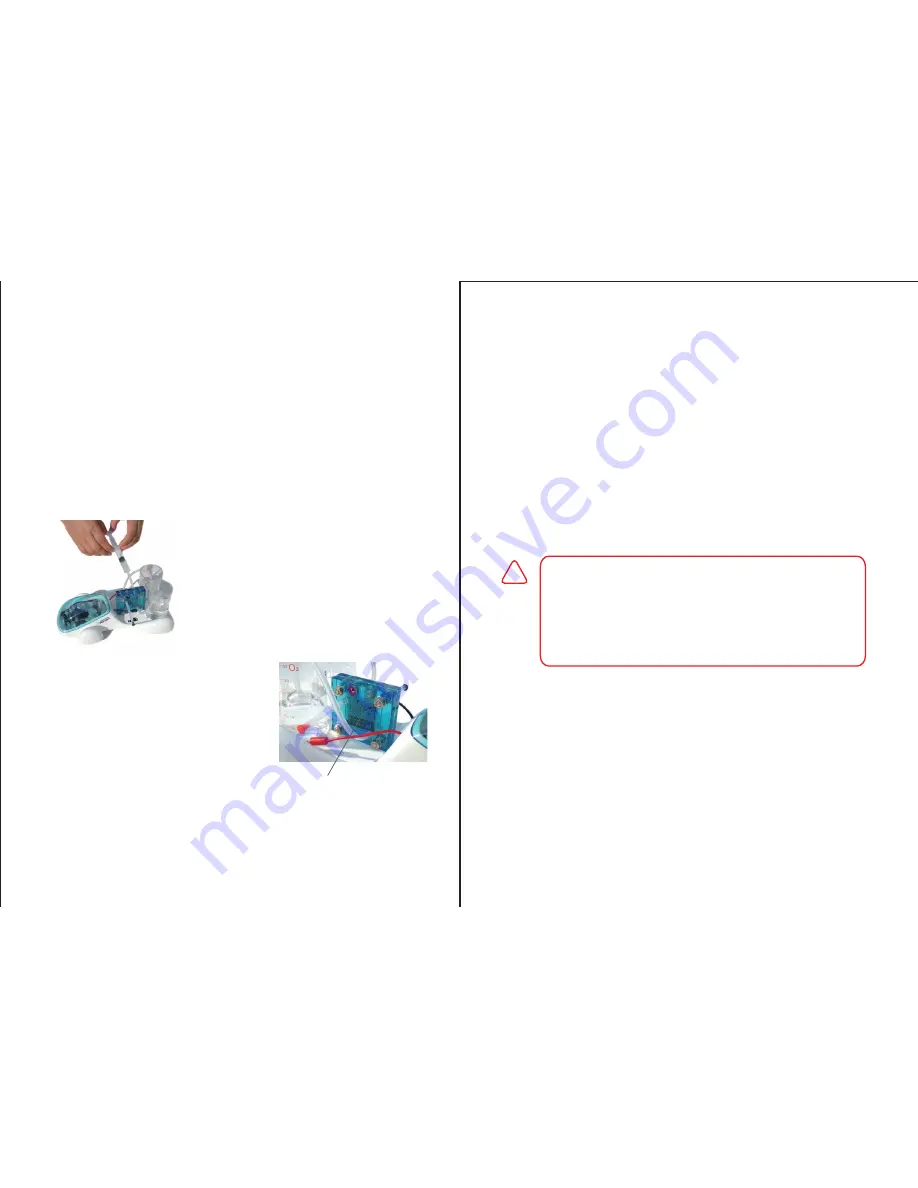
Step 4:
Step 5:
Insert the hydrogen and oxygen outer storage cylinders (H) into the round slots located on
the chassis (E). Fill the cylinders with distilled water upto the 20 mark on each of the outer
storage cylinders (H).
Insert the inner cylinders (G) into the outer cylinders (H) so that the inner cylinders (G) are
filled with water. There are two notches at the bottom of each of the inner cylinders (G).
These openings allow for gas to escape the inner cylinders (G) into the outer cylinders (H)
to limit the amount of gas stored. Make sure these openings are not blocked by the raised
plastic frame holding the inner cylinders (G). Push on the top of the inner cylinders (G) to
be sure they fit firmly onto the plastic rim located at the bottom of the outer storage cylin-
ders (H).
Attach the long tubes (I) to the top of the inner cylinders (G). Attach the long tube coming
from the hydrogen storage cylinder to the lower nozzle of the hydrogen side of the fuel cell.
Attach the long tube coming from the oxygen storage cylinder to the lower nozzle of the
oxygen side of the fuel cell.
Step 6:
water
Good ion conductivity is critical to the perfor-
mance of the fuel cell. In order to ensure
good conductivity, the fuel cell’s membrane
needs to be properly humidified.
In order to hydrate the fuel cell, complete the following
points:
Use the syringe (A) to suck distilled water (purchased
separately) into the syringe. Once the syringe is filled with
distilled water, place the syringe into the top nozzle on the
oxygen side of the fuel cell (C) and proceed to push water
into the oxygen side of the fuel cell (marked O
2
) until you
see water passing through the chamber in front of the
screen and out the lower nozzle. Leave the fuel cell for 5
to 10 minutes to fully hydrate.
5. Electrolysis: create Hydrogen from water
Electrolysis is the process of converting electrical energy to chemical energy. When an electrical
charge is applied to water, the charge breaks the chemical bond between hydrogen and oxygen
and creates charged particles called ions. In this case, positively charged hydrogen ions and
negatively charged ions are formed. An electrolyzer has 2 electrodes where the ions form. One
electrode, called the anode, is positively charged, and attracts negatively charged ions. The other
electrode is called the cathode and attracts the positively charged hydrogen ions.
Reversible fuel cells can be used to perform electrolysis. In a fuel cell, the electrolyte is part of the
membrane assembly. When a current is applied to a fuel cell, it will electrolyze water producing
hydrogen on the cathode side and oxygen on the anode side.
Note:
Follow these next instructions only after you have completed all the steps in Chapter 4:
“Assembly of the kit ”. Make sure the fuel cell has been hydrated by injecting water using the
syringe before proceeding to electrolysis.
!
Warning:
Using non-distilled water damages the electrodes of the fuel cells. Fuel cells use
nano-scale or carbon supported platinum as a catalyst, and these particles are very sensitive to
impurities found in non-distilled water.
For the purposes of this hydrocar kit, high quality drinking water or tap water with low mineral
content can also be used - however usability of the kit will inevitably be shortened.
The Fuel Cell must ONLY be hydrated through the O
2
side and NOT through the H
2
side, failure
to do so will result in the blockage of hydrogen flow.
Step 1:
Step 2:
Insert one end with the banana plug of the red cable into the red jack of the solar panel, the
other end with the banana plug into the fuel cell.
Insert one end with the banana plug of the black cable into the black jack of the solar panel,
the other end with the banana plug into the fuel cell.
a. Using Solar Panel for electrolysis
The Hydrocar kit can use a small solar photovoltaic cell for the electrolysis process as a way to capture
renewable energy from the sun. Please follow the instructins below:







































