22
23
V. Others
* The InBody370S is manufactured according to the quality management procedure of InBody. InBody complies with the ISO9001 and
ISO13485 which are international quality management systems.
* This equipment satisfies the IEC60601-1 (EN60601-1), an international safety standard for electronic medical equipment. This equipment
also satisfies the IEC60601-1-2 (EN60601-1-2), an international standard for electromagnetic conformity.
A. Exterior and Functions
The following are the names and functions of each part of the InBody.
* Please inspect each component of the InBody370S for damage prior to installation.
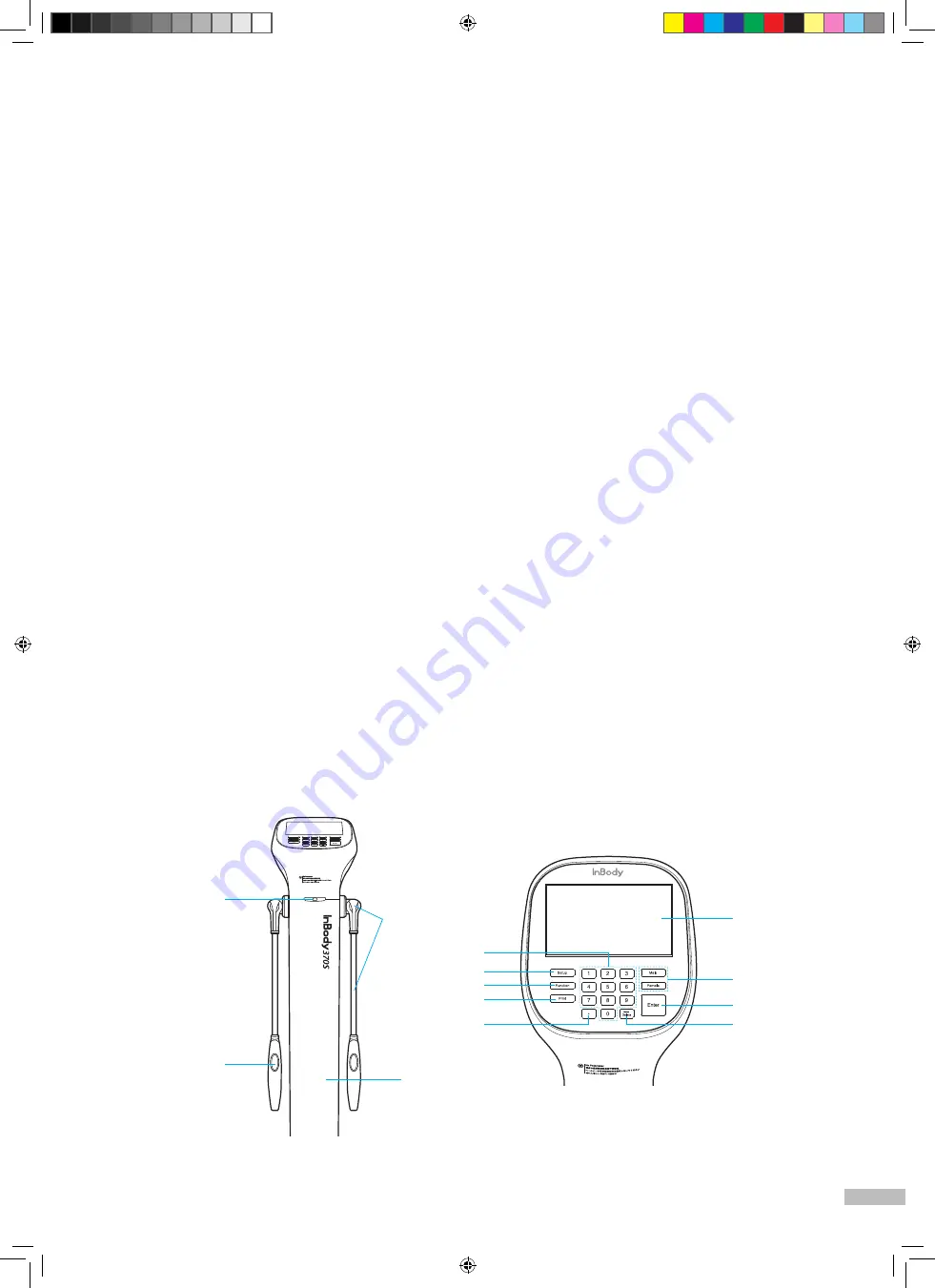
1. Upper Part
❶
Status LED: The LED emits a blue light when the InBody370S is turned on.
❷
Hand electrode: Examinee holds the hand electrode so that the 4 fingers wrap the surface of the
bottom hand electrode while the thumb is placed on the oval electrode.
❸
Hand electrode joint and handle: Supports the hand electrode and encloses the wiring for the electrode.
❹
Body: Connects the upper part of the equipment to the lower part.
❺
LCD screen: Shows each stage of the test, instructions, test results, etc. You can touch the screen to
input the data required for the test, configure settings, or view test results.
❻
Number keypad: Used for inputting age, height, and other number-based data.
❼
Setup button : Used for entering ‘Settings’ under the Administrator Menu when no one is on the footplate.
❽
Function button: Used for entering ‘Troubleshooting’ under the Administrator Menu when no one is on
the footplate.
❾
Print button: Used for printing the test results.
❿
Decimal point button: Used for inputting the decimal point in ID, height, age, or weight.
⓫
Gender buttons: Used for selecting gender (Male or Female).
⓬
Enter button: Used to finish inputting data or to save changes in Administrator Menu.
⓭
Delete button: Used for deleting inputted data.
❶
❸
❹
❷
❻
❼
❽
❾
❿
❺
⓫
⓬
⓭
370S_manual_Eng_C_181224.indd 23
2018. 12. 24. 오후 7:29