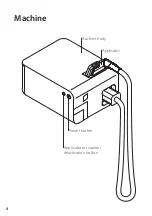
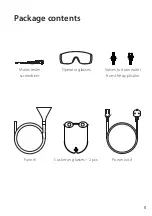
8
Precautions
1. Use this system strictly for its intended purpose as described in this
User Manual. Follow your doctor’s advice. Any other use of this device
is inappropriate and can be dangerous. The manufacturer is not liable
for any damage incurred due to device improper or unreasonable use
or if it was connected to the electricity mains incompatible with safety
requirements.
2. It is recommended to use the device with a quality 2,000 W mains
voltage stabilizer.
3. Prior to plugging the product into the mains outlet, make sure that the
mains voltage and frequency comply with the electrical specifications
shown on the specification plate on the back of the product.
4. The device must be used in a room temperature of 10 to 28o C.
5. If the device was exposed to conditions other than the acceptable
operating conditions, please wait until it restores its normal condition
before using it.
6. In case of the ingress of any extraneous liquids into the device, it must be
allowed to dry before use.
7. Do not use tap or purified water or other liquids not recommended by
the developer for the device system cooling.
8. Use the device in a clean environment free of dirt, dust, animal hair, etc.
9. The device should not be used near flammable substances or flammable
anesthetic mixtures with air, oxygen or premixed nitrous oxide.
10. It is forbidden to make any changes to the device design.
11. The device may heat up when used.
12. Do not place the device on top of any other equipment or under it. Do
not open the applicator body as it is not intended to be serviced.
13. Proper operation of this device may be impaired if you fail to follow these
User Manual instructions and/or do not use original spare parts.
14. Do not place the device on furniture and/or materials able to accumulate
static electricity.
15. While working with the device, the operator and the customer are
advised to avoid using things accumulating static electricity.
16. This device should be used intermittently in “1,000 pulses/10 min break”