User manual
IRISBOND
Hiru
See it Possible
www.irisbond.com
This declaration is signed on behalf
of Irisbond Crowdbonding, Ltd in
San Sebastián, on the 30th of April,
2021, by Eduardo Jauregui, CEO.
Declaration of conformity (MDR)
MANUFACTURER
IRISBOND CROWDBONDING, SL
ES-B75091058
AVENIDA DE TOLOSA, 75 - 2º
San Sebastián, 20018
Guipúzcoa, Spain
+34 9434 96 622
http://www.irisbond.com
REFERENCE
OSKOL WINDOWS
PRODUCT
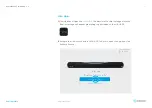
This product is composed by the following elements:
•
Medical device; Eye tracking system HIRU.
•
Case to bundle the Irisbond HIRU eye tracker and the Surface Pro tablet (TPU material has PASSED skin sensitization
and cytotoxicity tests in accordance with ISO 10993-5 and 10993-10).
We, Irisbond Crowdbonding Ltd, declare that the product listed below has been designed and manufactured in conformity
with the Directive (UE) 2017/745:
APPLICABLE DIRECTIVE
REGULATION (EU) 2017/745 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 5 April 2017 on medical
devices, MRD
, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and
repealing Council Directive 90/385/EEC.
The aim of this declaration is a Class I Medical Device and is in conformity with the following directives:
HARMONIZED LEGISLATION
HIRU:
EN 55032: 2015 / AC: 2016 / A11: 2020
EN 55035: 2017
UNE-EN 62471-1:2009
FCC CFR 47, Part 15, Subpart B (10-1-15 Edition)
ICES-003 Issue 6: 2016
OSKOL Windows:
ISO 10993-5
ISO 10993-10
TEST CERTIFICATES
65321IEM.001
65321REM.001
65321REM.002
2251989-PHO-21-018A
The following harmonized standards and technical specifications have been applied: