www.kawemed.com
19
Type BF device according to DIN IEC 601 Part 1 /
VDE 0750 Part 1
Heed the user‘s manual
Warning!
Separate disposal of electric and electronic
devices
Manufacturer
Date of manufacture
LOT
Lot code
Complies with relevant EU guidelines
IP41
Protected against water droplets and solid
matter with a diameter of greater than or equal
to 1.0mm
REF
Product reference number
SN
Series number
Temperature limit
GOST-R certification of exports to Russia
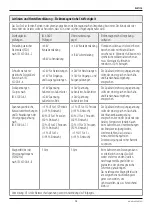
7. Contraindications/side effects and
preventative measures
IMPORTANT! Vital information:
Consult your physician before using this device to receive an
individualised treatment plan. Tap water iontophoresis may
not be performed under the following circumstances or if the
patient has one of the following conditions (contraindications):
- implanted electronic devices (i.e. pacemaker)
- pregnancy
- metal implants in the areas of the body through which the
current is conducted (arms or legs)
- metal-containing intrauterine devices (contraceptive coils)
when treating the feet
- large-sized skin defects that cannot be covered with
Vaseline or isolating bandages/wraps
- heart murmurs or insensitivity to pain.
The device may only be installed and operated in accordance
with the instructions given in the user’s manual. Radios,
mobile telephones or other similar devices that may affect the
device must be placed at least 2 metres away from the device.
The device may not be used by unsupervised children and
must be stored out of reach of children. The cables pose a risk
of strangulation. Small parts may become loose and could be
swallowed or inhaled.
Only use standard accessories provided by KaWe. The use of
other cables, charging devices, electrodes, water trays etc. is
not allowed. The use of non-standard parts can influence the
EMC interference immunity and electrical safety of the unit.
Simultaneous treatment with another high-frequency surgical
instrument may cause skin burns under or near the electrodes.
The operation of the iontophoresis device within close range
(approx. 1 m) of a short-wave or a microwave therapy unit can
cause fluctuations in the output readings of the iontophoresis
device and therefore, should be avoided.
Never perform treatments during thunderstorms. If necessary,
immediately discontinue the treatment and turn off the unit.
The maximum current and the maximum voltage are limited
by safety values that are determined by the regulations for
electrical medical devices, thereby eliminating the risk of harm
to the patient.
Electrical shocks are primarily caused by contact failures
between the connector cables and the electrodes. Over time,
transfer resistance occurs between the electrodes and the
connector cables due to the direct contact with water. This
can be avoided by disconnecting the cable on the electrode
just before beginning treatment and then reinserting it with a
twisting motion. The friction thereby produced creates a clean
metal-to-metal contact which is essential for a constant flow
of current. This practically eliminates uncomfortable current
peaks that could otherwise be felt by the patient.
Persons with prosthetics must remove these before treatment.
They must also consult a doctor before using this device.
It is essential that the patient ensure that the success of the
treatment is not affected by any type of disturbance. Due to
this reason as well for electrical safety reasons, it must be
ensured that no children or house pets are allowed in the room
at the time of treatment.
The patient should be aware of the fact that – especially
during underarm perspiration treatment – any rash movement
during the treatment can alter the surface on the body being
used for the current transfer. As a result, the current load on
the affected body part may exceed the allowed value, which
although it cannot cause actual harm, could potentially
cause discomfort, skin irritation and/or slight burns. Such
ENGLISH